

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50103860 2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-methyl-3H-benzoimidazole-5-carbonyl]-amino}-3-phosphono-propionic acid::2-{[2-(6-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-methyl-3H-benzoimidazole-5-carbonyl]-amino}-3-phosphono-propionic acid::CHEMBL421708
SMILES: Cn1c(Cc2nc3ccc(cc3[nH]2)C(N)=O)nc2ccc(cc12)C(=O)NC(CP(O)(O)=O)C(O)=O
InChI Key: InChIKey=PEXFKSJDXSBOLP-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM50103860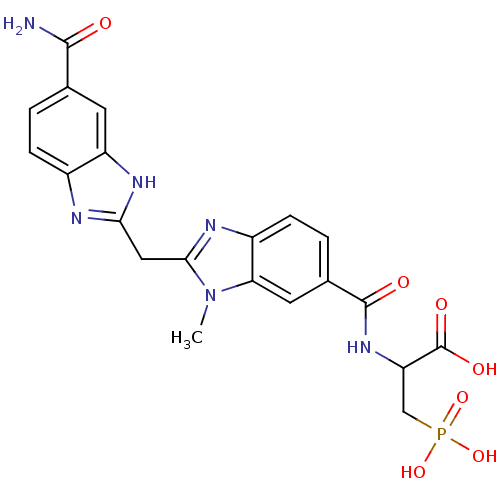 (2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 wild-type reverse transcriptase. | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1007/s00044-005-0131-1 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM50103860 (2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of HCV serine protease NS3/NS4A in the presence of EDTA. | Bioorg Med Chem Lett 12: 3129-33 (2002) BindingDB Entry DOI: 10.7270/Q25D8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM50103860 (2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase G190A | Eur J Med Chem 164: 576-601 (2019) Article DOI: 10.1007/s00044-005-0131-1 BindingDB Entry DOI: 10.7270/Q2RR224D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM50103860 (2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of HCV serine protease NS3/NS4A in the presence of EDTA. | Bioorg Med Chem Lett 12: 3129-33 (2002) BindingDB Entry DOI: 10.7270/Q25D8R64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM50103860 (2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against hepatitis C virus (HCV) NS3 protease in the absence of Zn2+ | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM50103860 (2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||