

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50104552 (S)-1-(2-aza-bicyclo[2.2.2]octan-2-yl)-2-(2-(3,5-dimethylphenyl)-3-(1-(2-(pyridin-4-yl)ethylamino)propan-2-yl)-1H-indol-5-yl)-2-methylpropan-1-one::1-(2-Aza-bicyclo[2.2.2]oct-2-yl)-2-{2-(3,5-dimethyl-phenyl)-3-[(S)-1-methyl-2-(2-pyridin-4-yl-ethylamino)-ethyl]-1H-indol-5-yl}-2-methyl-propan-1-one::CHEMBL314260
SMILES: C[C@H](CNCCc1ccncc1)c1c([nH]c2ccc(cc12)C(C)(C)C(=O)N1CC2CCC1CC2)-c1cc(C)cc(C)c1
InChI Key: InChIKey=GGVFLXSCFPVLMS-WPCLDFDBSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gonadotropin releasing hormone 1 (GnRHR1) (Homo sapiens (Human)) | BDBM50104552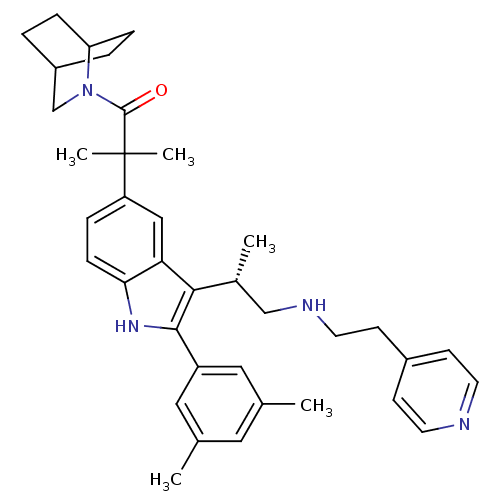 ((S)-1-(2-aza-bicyclo[2.2.2]octan-2-yl)-2-(2-(3,5-d...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro functional antagonism via inhibition of GnRH-stimulated phosphatidylinositol (PI) hydrolysis in cloned Chinese hamster ovary (CHO) cells sta... | Bioorg Med Chem Lett 11: 2597-602 (2001) BindingDB Entry DOI: 10.7270/Q2NV9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50104552 ((S)-1-(2-aza-bicyclo[2.2.2]octan-2-yl)-2-(2-(3,5-d...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition towards human pituitary gonadotropin-releasing hormone receptor using [125I]-buserelin. | Bioorg Med Chem Lett 11: 2597-602 (2001) BindingDB Entry DOI: 10.7270/Q2NV9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50104552 ((S)-1-(2-aza-bicyclo[2.2.2]octan-2-yl)-2-(2-(3,5-d...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity at rat GnRH receptor by whole cell binding assay | J Med Chem 51: 3331-48 (2008) Article DOI: 10.1021/jm701249f BindingDB Entry DOI: 10.7270/Q2KD1XQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||