

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50112183 CHEMBL3608684::US10214533, Compound 1::US9969738, 1
SMILES: Cc1nsc(n1)-c1nnc2CN(CCn12)C(=O)c1ccc(F)cc1
InChI Key: InChIKey=UHNVQJPBRBMNNN-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50112183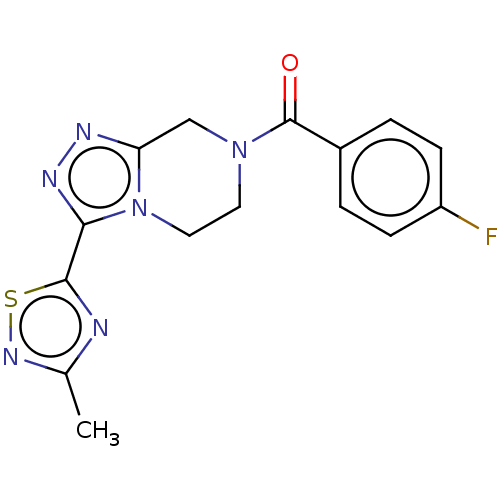 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Binding affinity to human recombinant NK3R by radioligand binding assay | ACS Med Chem Lett 6: 736-40 (2015) BindingDB Entry DOI: 10.7270/Q2TQ639V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor(NK3R) (Homo sapiens (Human)) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description NK-2/NK-3: The affinity of compounds of the invention for the NK-2 receptor was evaluated in CHO recombinant cells which express the human NK-2 recep... | J Med Chem 52: 1935-42 (2009) BindingDB Entry DOI: 10.7270/Q2N58PQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor(NK3R) (Homo sapiens (Human)) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description NK-2/NK-3: The affinity of compounds of the invention for the NK-2 receptor was evaluated in CHO recombinant cells which express the human NK-2 recep... | J Med Chem 52: 1935-42 (2009) BindingDB Entry DOI: 10.7270/Q2N58PQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Rattus norvegicus) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Binding affinity to rat NK3R | ACS Med Chem Lett 6: 736-40 (2015) BindingDB Entry DOI: 10.7270/Q2TQ639V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurokinin 2 receptor (Homo sapiens (Human)) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The affinity of compounds of the invention for the NK-2 receptor was evaluated in CHO recombinant cells which express the human NK-2 receptor. Membra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurokinin 1 receptor (Homo sapiens (Human)) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The affinity of compounds of the invention for the NK-1 receptor was evaluated in CHO recombinant cells which express the human NK-1 receptor. Membra... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurokinin 2 receptor (Homo sapiens (Human)) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description NK-2/NK-3: The affinity of compounds of the invention for the NK-2 receptor was evaluated in CHO recombinant cells which express the human NK-2 recep... | J Med Chem 52: 1935-42 (2009) BindingDB Entry DOI: 10.7270/Q2N58PQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurokinin 1 receptor (Homo sapiens (Human)) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description NK-1: The affinity of compounds of the invention for the NK-1 receptor was evaluated in CHO recombinant cells which express the human NK-1 receptor. ... | J Med Chem 52: 1935-42 (2009) BindingDB Entry DOI: 10.7270/Q2N58PQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | ACS Med Chem Lett 6: 736-40 (2015) BindingDB Entry DOI: 10.7270/Q2TQ639V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Antagonist activity against human recombinant NK3R expressed in CHO cells by aequorin functional assay | ACS Med Chem Lett 6: 736-40 (2015) BindingDB Entry DOI: 10.7270/Q2TQ639V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | ACS Med Chem Lett 6: 736-40 (2015) BindingDB Entry DOI: 10.7270/Q2TQ639V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
OGEDA SA US Patent | Assay Description The determination of plasma protein binding (PPB) of a compound is enabled by equilibrium dialysis, an accepted and standard method for reliable esti... | US Patent US10214533 (2019) BindingDB Entry DOI: 10.7270/Q21J9D35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) | ACS Med Chem Lett 6: 736-40 (2015) BindingDB Entry DOI: 10.7270/Q2TQ639V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 6: 736-40 (2015) BindingDB Entry DOI: 10.7270/Q2TQ639V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Inhibition of human ERG | ACS Med Chem Lett 6: 736-40 (2015) BindingDB Entry DOI: 10.7270/Q2TQ639V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50112183 (CHEMBL3608684 | US10214533, Compound 1 | US9969738...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | ACS Med Chem Lett 6: 736-40 (2015) BindingDB Entry DOI: 10.7270/Q2TQ639V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||