

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50112697 (S)-2-Benzo[1,3]dioxol-5-yl-6-isopropoxy-4-(4-methoxy-phenyl)-2H-chromene-3-carboxylic acid::2-Benzo[1,3]dioxol-5-yl-6-isopropoxy-4-(4-methoxy-phenyl)-2H-chromene-3-carboxylic acid::CHEMBL264543
SMILES: COc1ccc(cc1)C1=C([C@@H](Oc2ccc(OC(C)C)cc12)c1ccc2OCOc2c1)C(O)=O
InChI Key: InChIKey=BATSJXIVNKJCKQ-SANMLTNESA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EDNRA (RAT) | BDBM50112697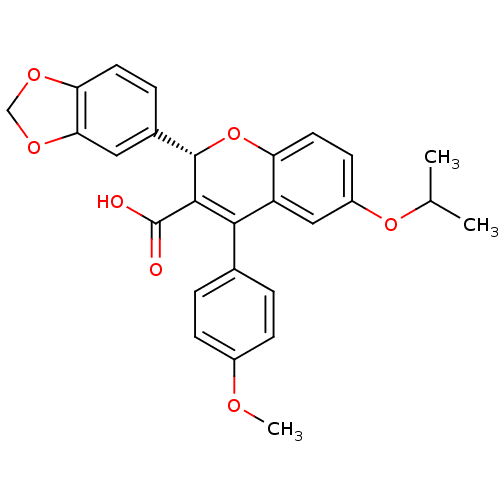 ((S)-2-Benzo[1,3]dioxol-5-yl-6-isopropoxy-4-(4-meth...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for endothelin A receptor by inhibition of [125I]-ET-1 binding in rat aorta smooth muscle cells | J Med Chem 45: 2041-55 (2002) BindingDB Entry DOI: 10.7270/Q24J0DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| EDNRA (RAT) | BDBM50112697 ((S)-2-Benzo[1,3]dioxol-5-yl-6-isopropoxy-4-(4-meth...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Ability to displace [125I]-ET-1 from the rat endothelin A receptor expressed in rat aorta smooth muscle cells. | J Med Chem 47: 2750-60 (2004) Article DOI: 10.1021/jm031041j BindingDB Entry DOI: 10.7270/Q26T0M3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor ET-B (Sus scrofa) | BDBM50112697 ((S)-2-Benzo[1,3]dioxol-5-yl-6-isopropoxy-4-(4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to endothelin B receptor measured by inhibition of [125I]-ET-3 binding recombinant pig ET-B receptor expressed in COS-7 cells | J Med Chem 45: 2041-55 (2002) BindingDB Entry DOI: 10.7270/Q24J0DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||