

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50119786 1,8-di(2-isoquinoliniumyl)octane; with diiodide ions::2,2'-(octane-1,8-diyl)diisoquinolinium iodide::CHEMBL104265::CHEMBL1669486
SMILES: C(CCCC[n+]1ccc2ccccc2c1)CCC[n+]1ccc2ccccc2c1
InChI Key: InChIKey=GMXBRECDYFCFAU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50119786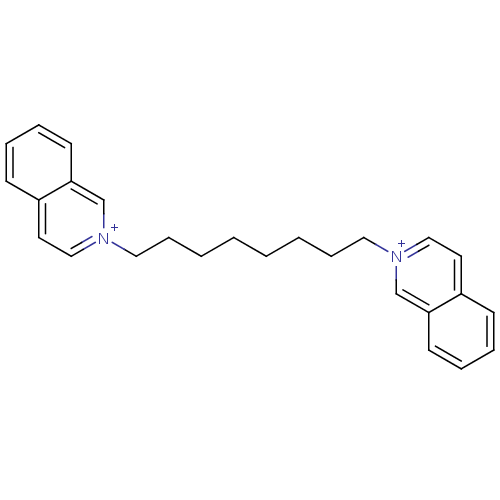 (1,8-di(2-isoquinoliniumyl)octane; with diiodide io...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence Curated by ChEMBL | Assay Description Dissociation constant for enzyme-inhibitor complex of human recombinant AChE by Lineweaver-Burk plot analysis | Eur J Med Chem 46: 811-8 (2011) Article DOI: 10.1016/j.ejmech.2010.12.011 BindingDB Entry DOI: 10.7270/Q2PR7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50119786 (1,8-di(2-isoquinoliniumyl)octane; with diiodide io...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence Curated by ChEMBL | Assay Description Dissociation constant for enzyme-inhibitor-substrate complex of human recombinant AChE by Lineweaver-Burk plot analysis | Eur J Med Chem 46: 811-8 (2011) Article DOI: 10.1016/j.ejmech.2010.12.011 BindingDB Entry DOI: 10.7270/Q2PR7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor (Rattus norvegicus (Rat)) | BDBM50119786 (1,8-di(2-isoquinoliniumyl)octane; with diiodide io...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding affinity nicotinic acetylcholine receptor alpha-7 was evaluated by its ability to inhibit [3H]methyllycaconitine ([3H]-MLA) binding to rat br... | Bioorg Med Chem Lett 12: 3067-71 (2002) BindingDB Entry DOI: 10.7270/Q2Q81DNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor protein alpha-4/beta-2 subunit (Rattus norvegicus (Rat)) | BDBM50119786 (1,8-di(2-isoquinoliniumyl)octane; with diiodide io...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding affinity for nicotinic acetylcholine receptor alpha4-beta2 was evaluated by its ability to inhibit [3H]NIC binding to rat brain membranes | Bioorg Med Chem Lett 12: 3067-71 (2002) BindingDB Entry DOI: 10.7270/Q2Q81DNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50119786 (1,8-di(2-isoquinoliniumyl)octane; with diiodide io...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by Lineweaver-Burk plot analysis | Eur J Med Chem 46: 811-8 (2011) Article DOI: 10.1016/j.ejmech.2010.12.011 BindingDB Entry DOI: 10.7270/Q2PR7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM50119786 (1,8-di(2-isoquinoliniumyl)octane; with diiodide io...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE by Lineweaver-Burk plot analysis | Eur J Med Chem 46: 811-8 (2011) Article DOI: 10.1016/j.ejmech.2010.12.011 BindingDB Entry DOI: 10.7270/Q2PR7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||