

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(C)C[C@H](NC(=O)[C@H](C[Si](C)(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCCCN)C(O)=O
InChI Key: InChIKey=SXNKGENAHNQHOV-JNRWAQIZSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50124146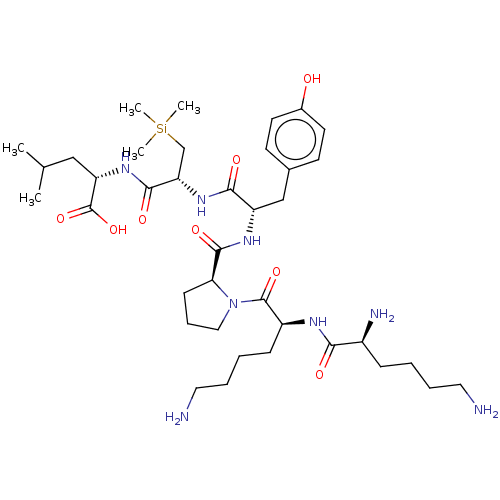 (CHEMBL3622801) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 169 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 receptor expressed in CHOK1 cells co-expressing Galphaq-RlucII(121)/Gbeta1/GFP10-Ggamma1 assessed as Galpha-q stimulat... | J Med Chem 58: 7785-95 (2015) BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50124146 (CHEMBL3622801) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 receptor expressed in CHOK1 cells co-expressing hNTS1-GFP10/RlucII-beta-arrestin 2 assessed as beta-arrestin2 recruitm... | J Med Chem 58: 7785-95 (2015) BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50124146 (CHEMBL3622801) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 in Sprague-Dawley rat ileum assessed as inhibition of carbachol-induced contraction | J Med Chem 58: 7785-95 (2015) BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50124146 (CHEMBL3622801) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 405 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr3-NT from human NTS2 receptor expressed in 1321N1 cell membranes incubated for 30 mins by gamma-counting based competitive ... | J Med Chem 58: 7785-95 (2015) BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50124146 (CHEMBL3622801) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr3-NT from human NTS1 receptor expressed in CHOK1 cell membranes incubated for 30 mins by gamma-counting based competitive r... | J Med Chem 58: 7785-95 (2015) BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||