

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50128853 16-Chloro-17,19-dihydroxy-4-methyl-3,7-dioxa-tricyclo[13.4.0.0*6,8*]nonadeca-1(19),9,11,15,17-pentaene-2,13-dione 13-[O-(2-oxo-2-pyrrolidin-1-yl-ethyl)-oxime]::CHEMBL314705
SMILES: C[C@@H]1C[C@H]2O[C@@H]2\C=C/C=C/C(/Cc2c(Cl)c(O)cc(O)c2C(=O)O1)=N\OCC(=O)N1CCCC1
InChI Key: InChIKey=VFJDDMTXWXGHIM-UYJTYAAOSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Rattus norvegicus) | BDBM50128853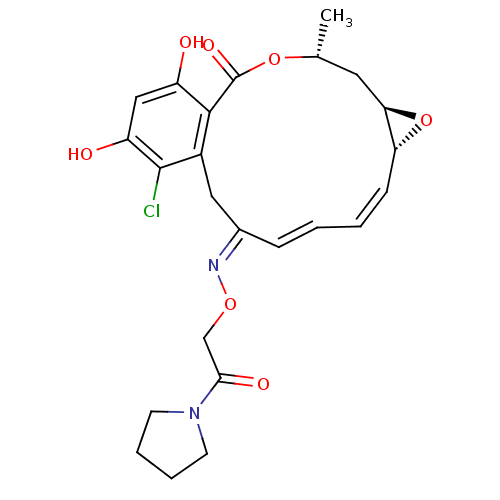 (16-Chloro-17,19-dihydroxy-4-methyl-3,7-dioxa-tricy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of v-Src tyrosine kinase autophosphorylation in SR3Y1 cells after 15 hr exposure | J Med Chem 46: 2534-41 (2003) Article DOI: 10.1021/jm030110r BindingDB Entry DOI: 10.7270/Q2H41QTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||