

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50131463 (6S,12R)-25-Chloro-11-cyclohexyl-21-oxa-3,9,12,18-tetraaza-tricyclo[20.4.0.0*5,9*]hexacosa-1(22),23,25-triene-4,10,13,19-tetraone::CHEMBL96376
SMILES: Clc1ccc2OCC(=O)NCCCCC(=O)N[C@H](C3CCCCC3)C(=O)N3CCC[C@H]3C(=O)NCc2c1
InChI Key: InChIKey=SDVDEFWBLCBKCC-SQJMNOBHSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50131463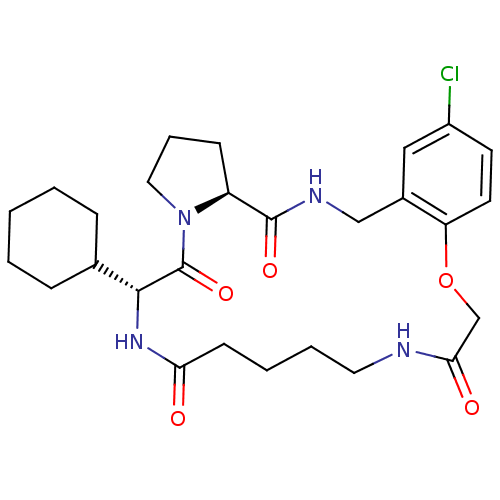 ((6S,12R)-25-Chloro-11-cyclohexyl-21-oxa-3,9,12,18-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of thrombin | J Med Chem 54: 1961-2004 (2011) Article DOI: 10.1021/jm1012374 BindingDB Entry DOI: 10.7270/Q28C9XDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131463 ((6S,12R)-25-Chloro-11-cyclohexyl-21-oxa-3,9,12,18-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thrombin IIa | Bioorg Med Chem Lett 13: 2781-4 (2003) BindingDB Entry DOI: 10.7270/Q2GF0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50131463 ((6S,12R)-25-Chloro-11-cyclohexyl-21-oxa-3,9,12,18-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Tissue plasminogen activator | Bioorg Med Chem Lett 13: 2781-4 (2003) BindingDB Entry DOI: 10.7270/Q2GF0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin-1 (Homo sapiens (Human)) | BDBM50131463 ((6S,12R)-25-Chloro-11-cyclohexyl-21-oxa-3,9,12,18-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human trypsin | Bioorg Med Chem Lett 13: 2781-4 (2003) BindingDB Entry DOI: 10.7270/Q2GF0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||