

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50139925 CHEMBL3764246::US10577361, E37
SMILES: Cc1nc(no1)-c1ccc(cc1)-c1nnc(SCCCN2CCOC(C2)c2ccc(C)cc2)n1C
InChI Key: InChIKey=WQOCUOYKDKZARU-UHFFFAOYSA-N
Data: 8 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50139925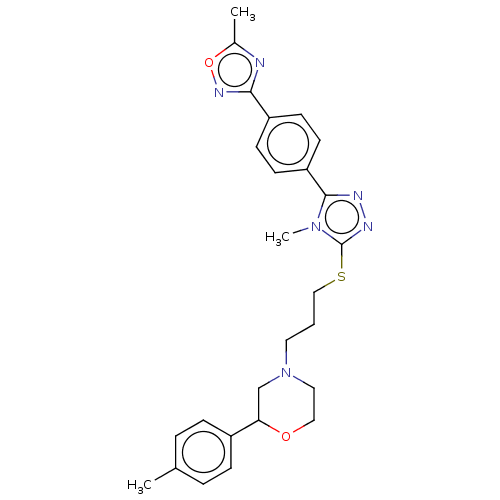 (CHEMBL3764246 | US10577361, E37) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay | Bioorg Med Chem Lett 26: 1329-32 (2016) BindingDB Entry DOI: 10.7270/Q2HM5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50139925 (CHEMBL3764246 | US10577361, E37) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D3 receptor after 45 mins by microplate scintillation counting analysis | Bioorg Med Chem Lett 26: 1329-32 (2016) BindingDB Entry DOI: 10.7270/Q2HM5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50139925 (CHEMBL3764246 | US10577361, E37) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 85.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INDIVIOR UK LIMITED US Patent | Assay Description [3H]-Spiperone Binding Assay at hD3 and hD4 recombinant receptors CHO cells transiently transfected with human dopamine type 3 or 4 receptors (CHO-hD... | US Patent US10577361 (2020) BindingDB Entry DOI: 10.7270/Q2GQ715H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50139925 (CHEMBL3764246 | US10577361, E37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Agonist activity at human dopamine D3 receptor expressed in CHO cells after 90 mins by [35S]-GTPgamma S assay | Bioorg Med Chem Lett 26: 1329-32 (2016) BindingDB Entry DOI: 10.7270/Q2HM5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50139925 (CHEMBL3764246 | US10577361, E37) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in bactosome using 7BQ as substrate | Bioorg Med Chem Lett 26: 1329-32 (2016) BindingDB Entry DOI: 10.7270/Q2HM5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50139925 (CHEMBL3764246 | US10577361, E37) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Displacement of [125I]-7-OH-PIPAT from rat brain dopamine D2 receptor after 45 mins by microplate scintillation counting analysis | Bioorg Med Chem Lett 26: 1329-32 (2016) BindingDB Entry DOI: 10.7270/Q2HM5B83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50139925 (CHEMBL3764246 | US10577361, E37) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INDIVIOR UK LIMITED US Patent | Assay Description CHO cells stably expressing human dopamine receptor type 2, long variant (hD2L), coupled to Gα16 protein (CHO-Gα16-hD2L) were seeded into b... | US Patent US10577361 (2020) BindingDB Entry DOI: 10.7270/Q2GQ715H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| d2 (Homo sapiens (Human)) | BDBM50139925 (CHEMBL3764246 | US10577361, E37) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INDIVIOR UK LIMITED US Patent | Assay Description CHO cells stably expressing human dopamine receptor type 2, long variant (hD2L), coupled to Gα16 protein (CHO-Gα16-hD2L) were re-suspended ... | US Patent US10577361 (2020) BindingDB Entry DOI: 10.7270/Q2GQ715H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||