

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50140707 5-(3-chloro-4-methylphenyl)-3-phenyl-(3R,3aS,6aR)-spiro[perhydrofuro[3,4-c]pyrrole-1,2''-(2'',3''-dihydro-1''H-indene)]-1'',3'',4,6-tetraone::CHEMBL26946::CHEMBL281883
SMILES: Cc1ccc(cc1Cl)-n1c(O)c2C(OC3(C(=O)c4ccccc4C3=O)c2c1O)c1ccccc1
InChI Key: InChIKey=WZLJTSSHRBYEDZ-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenylalanyl-tRNA synthetase alpha chain (Streptococcus pyogenes serotype M18) | BDBM50140707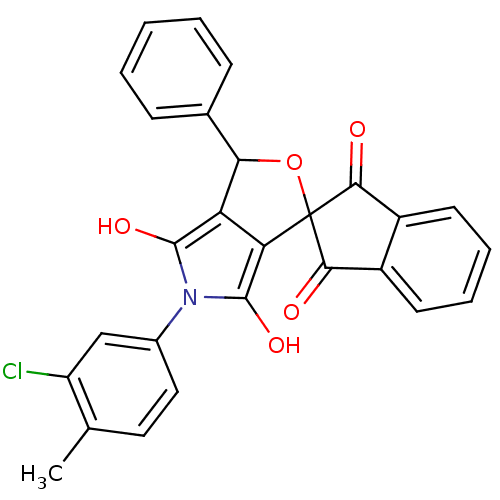 (5-(3-chloro-4-methylphenyl)-3-phenyl-(3R,3aS,6aR)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against phenylalanyl-tRNA synthetase from Enterococcus faecalis | Bioorg Med Chem Lett 14: 1339-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.081 BindingDB Entry DOI: 10.7270/Q2PR7VDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanyl-tRNA synthetase mitochondrial (Homo sapiens (Human)) | BDBM50140707 (5-(3-chloro-4-methylphenyl)-3-phenyl-(3R,3aS,6aR)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human phenylalanyl-tRNA synthetase was determined | Bioorg Med Chem Lett 14: 1339-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.081 BindingDB Entry DOI: 10.7270/Q2PR7VDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanyl-tRNA synthetase alpha chain (Streptococcus pyogenes serotype M18) | BDBM50140707 (5-(3-chloro-4-methylphenyl)-3-phenyl-(3R,3aS,6aR)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against phenylalanyl-tRNA synthetase from Staphylococcus aureus | Bioorg Med Chem Lett 14: 1339-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.081 BindingDB Entry DOI: 10.7270/Q2PR7VDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanyl-tRNA synthetase alpha chain (Streptococcus pyogenes serotype M18) | BDBM50140707 (5-(3-chloro-4-methylphenyl)-3-phenyl-(3R,3aS,6aR)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against phenylalanyl-tRNA synthetase from Enterococcus faecalis | Bioorg Med Chem Lett 14: 1339-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.081 BindingDB Entry DOI: 10.7270/Q2PR7VDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanyl-tRNA synthetase alpha chain (Streptococcus pyogenes serotype M18) | BDBM50140707 (5-(3-chloro-4-methylphenyl)-3-phenyl-(3R,3aS,6aR)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against phenylalanyl-tRNA synthetase from Staphylococcus aureus | Bioorg Med Chem Lett 14: 1339-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.081 BindingDB Entry DOI: 10.7270/Q2PR7VDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanyl-tRNA synthetase mitochondrial (Homo sapiens (Human)) | BDBM50140707 (5-(3-chloro-4-methylphenyl)-3-phenyl-(3R,3aS,6aR)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against phenylalanyl-tRNA synthetase from Enterococcus faecalis | Bioorg Med Chem Lett 14: 1339-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.081 BindingDB Entry DOI: 10.7270/Q2PR7VDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||