

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50140715 3-(4-chlorophenyl)-5-(4-chloro-3-trifluoromethylphenyl)-(3R,3aS,6aR)-spiro[perhydrofuro[3,4-c]pyrrole-1,2'-(2',3'-dihydro-1'H-indene)]-1',3',4,6-tetraone::CHEMBL282736
SMILES: Oc1c2C(OC3(C(=O)c4ccccc4C3=O)c2c(O)n1-c1ccc(Cl)c(c1)C(F)(F)F)c1ccc(Cl)cc1
InChI Key: InChIKey=FILYRSGYNRLLDF-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenylalanyl-tRNA synthetase mitochondrial (Homo sapiens (Human)) | BDBM50140715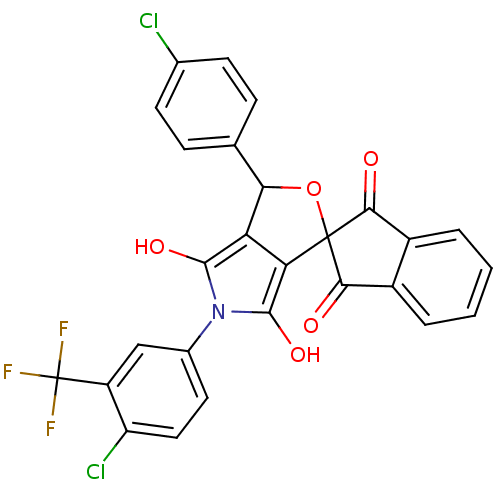 (3-(4-chlorophenyl)-5-(4-chloro-3-trifluoromethylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human phenylalanyl-tRNA synthetase was determined | Bioorg Med Chem Lett 14: 1339-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.081 BindingDB Entry DOI: 10.7270/Q2PR7VDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanyl-tRNA synthetase alpha chain (Streptococcus pyogenes serotype M18) | BDBM50140715 (3-(4-chlorophenyl)-5-(4-chloro-3-trifluoromethylph...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human phenylalanyl-tRNA synthetase was determined | Bioorg Med Chem Lett 14: 1339-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.081 BindingDB Entry DOI: 10.7270/Q2PR7VDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanyl-tRNA synthetase alpha chain (Streptococcus pyogenes serotype M18) | BDBM50140715 (3-(4-chlorophenyl)-5-(4-chloro-3-trifluoromethylph...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against phenylalanyl-tRNA synthetase from Staphylococcus aureus | Bioorg Med Chem Lett 14: 1339-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.081 BindingDB Entry DOI: 10.7270/Q2PR7VDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||