

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50142741 (E)-6-[1-(4-Chloro-benzyl)-1H-pyrrol-2-yl]-2,4-dioxo-hex-5-enoic acid::CHEMBL299873
SMILES: OC(=O)C(=O)CC(=O)C=Cc1cccn1Cc1ccc(Cl)cc1
InChI Key: InChIKey=FOSFBOADBRDSKM-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50142741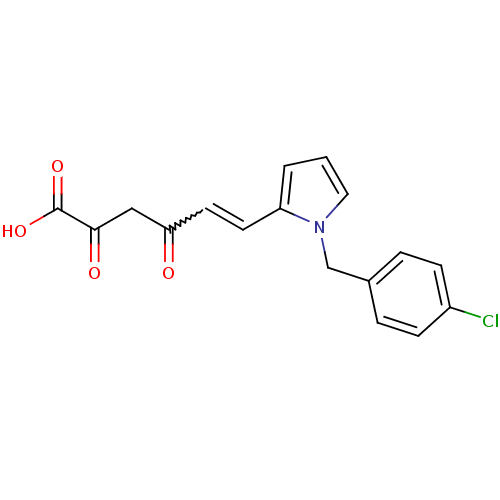 ((E)-6-[1-(4-Chloro-benzyl)-1H-pyrrol-2-yl]-2,4-dio...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma 'La Sapienza' Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 integrase. | Bioorg Med Chem Lett 14: 1745-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.037 BindingDB Entry DOI: 10.7270/Q2B56J61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50142741 ((E)-6-[1-(4-Chloro-benzyl)-1H-pyrrol-2-yl]-2,4-dio...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of RNase H activity of recombinant HIV1 reverse transcriptase using poly(dC)-[3H]poly(rG) as substrate | J Med Chem 56: 8588-98 (2013) Article DOI: 10.1021/jm401040b BindingDB Entry DOI: 10.7270/Q2BZ690C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50142741 ((E)-6-[1-(4-Chloro-benzyl)-1H-pyrrol-2-yl]-2,4-dio...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of recombinant HIV1 integrase using 5'-end-labeled 21-mer double-stranded DNA as substrate after 60 mins by el... | J Med Chem 56: 8588-98 (2013) Article DOI: 10.1021/jm401040b BindingDB Entry DOI: 10.7270/Q2BZ690C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50142741 ((E)-6-[1-(4-Chloro-benzyl)-1H-pyrrol-2-yl]-2,4-dio...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma 'La Sapienza' Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 integrase. | Bioorg Med Chem Lett 14: 1745-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.037 BindingDB Entry DOI: 10.7270/Q2B56J61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||