

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCCCCCOCCCc1cnc[nH]1
InChI Key: InChIKey=DCYQKSJPAHNDBM-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50146129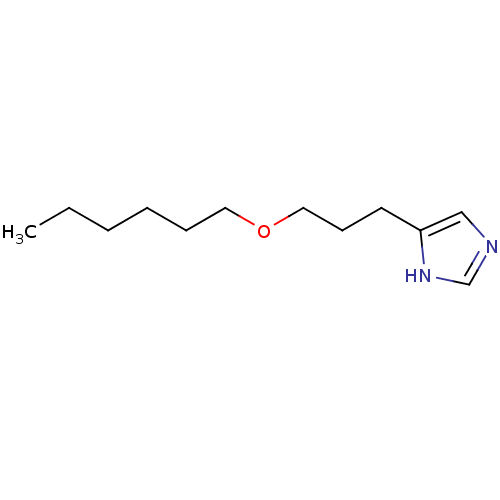 (4-(3-Hexyloxy-propyl)-1H-imidazole; compound with ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50146129 (4-(3-Hexyloxy-propyl)-1H-imidazole; compound with ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Functional Histamine H1 receptor antagonistic activity in vitro assay on guinea pig ileum | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50146129 (4-(3-Hexyloxy-propyl)-1H-imidazole; compound with ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146129 (4-(3-Hexyloxy-propyl)-1H-imidazole; compound with ...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Effect on specific [35S]-GTP-gammaS, Binding to HEK293 cell membranes expressing the human Histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50146129 (4-(3-Hexyloxy-propyl)-1H-imidazole; compound with ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Functional Histamine H3 receptor antagonistic activity in vitro assay on guinea pig ileum | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||