

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50158326 CHEMBL3781669::US10858316, Compound 3bg
SMILES: OC(=O)c1cc(c2ccccc2c1O)S(=O)(=O)Nc1ccc(Oc2ccc(Cl)cc2Cl)cc1
InChI Key: InChIKey=MHLNOPQXHVWPEC-UHFFFAOYSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50158326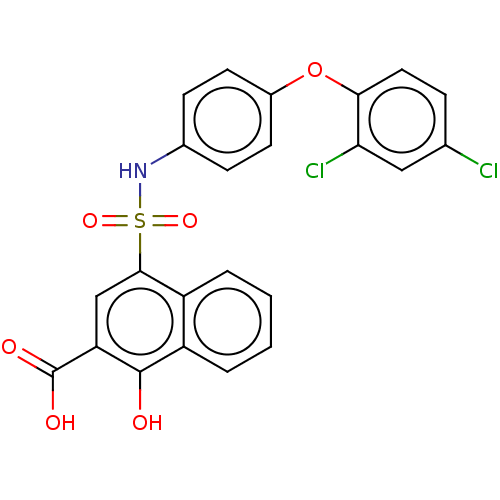 (CHEMBL3781669 | US10858316, Compound 3bg) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of His6-MBP tagged recombinant human Mcl-1 residues 172 to 327 expressed in Escherichia coli assessed as inhibition of interaction with Ba... | Eur J Med Chem 113: 273-92 (2016) BindingDB Entry DOI: 10.7270/Q2HX1FJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50158326 (CHEMBL3781669 | US10858316, Compound 3bg) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF MARYLAND, BALTIMORE US Patent | Assay Description Molecular modeling and SILCS functional group affinity mapping (FragMaps) of the Mcl-1 binding site indicated that the carboxylic acid of designed mo... | US Patent US10858316 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Bcl-X(L) (Homo sapiens (Human)) | BDBM50158326 (CHEMBL3781669 | US10858316, Compound 3bg) | PDB MMDB B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF MARYLAND, BALTIMORE US Patent | Assay Description Molecular modeling and SILCS functional group affinity mapping (FragMaps) of the Mcl-1 binding site indicated that the carboxylic acid of designed mo... | US Patent US10858316 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||