

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: OCc1ccc(s1)-c1cccnc1
InChI Key: InChIKey=XBSQDYZSJJZBOS-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50158933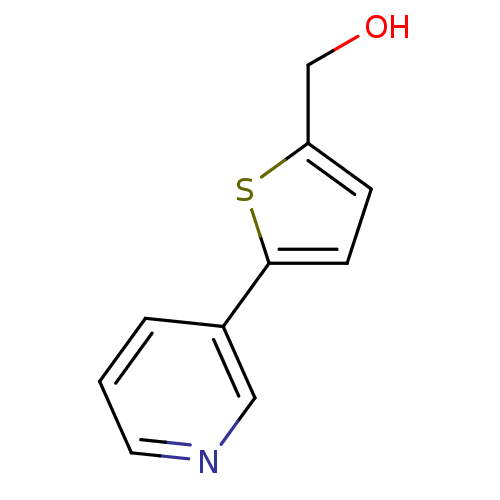 ((5-Pyridin-3-yl-thiophen-2-yl)-methanol | CHEMBL18...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Effect on coumarin 7-hydroxylation by human Cytochrome P-450 2A6 | J Med Chem 48: 224-39 (2005) Article DOI: 10.1021/jm049696n BindingDB Entry DOI: 10.7270/Q2T154DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50158933 ((5-Pyridin-3-yl-thiophen-2-yl)-methanol | CHEMBL18...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 3.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||