

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50163397 2-Furan-2-yl-5-[(1R,4R)-5-(5-methyl-isoxazol-3-ylmethyl)-2,5-diaza-bicyclo[2.2.1]hept-2-yl]-[1,2,4]triazolo[1,5-a][1,3,5]triazin-7-ylamine::CHEMBL360729
SMILES: Cc1cc(CN2C[C@H]3C[C@@H]2CN3c2nc(N)n3nc(nc3n2)-c2ccco2)no1
InChI Key: InChIKey=LINVQZKJPIIWRP-CHWSQXEVSA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine Receptors A2a (A2a) (Rattus norvegicus (rat)) | BDBM50163397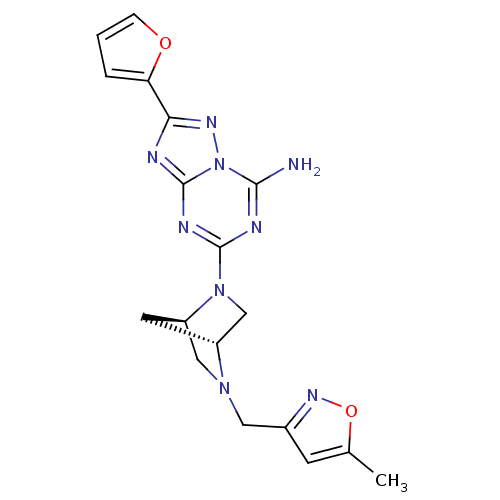 (2-Furan-2-yl-5-[(1R,4R)-5-(5-methyl-isoxazol-3-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]ZM-241385 binding to adenosine A2a receptor of rat brain tissue | J Med Chem 48: 2009-18 (2005) Article DOI: 10.1021/jm0498396 BindingDB Entry DOI: 10.7270/Q2NZ88FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||