

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50163728 (Benzyl-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-ethoxy]-benzyl}-amino)-acetic acid::2-((4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benzyl)(benzyl)amino)acetic acid::CHEMBL371120
SMILES: Cc1oc(nc1CCOc1ccc(CN(CC(O)=O)Cc2ccccc2)cc1)-c1ccccc1
InChI Key: InChIKey=KBXHBLXYBPVCLB-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50163728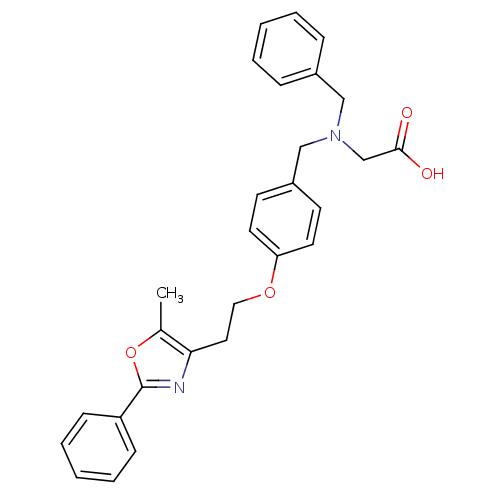 ((Benzyl-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-etho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Effective concentration against human Peroxisome proliferator activated receptor gamma | J Med Chem 48: 2248-50 (2005) Article DOI: 10.1021/jm0496436 BindingDB Entry DOI: 10.7270/Q2SX6CRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50163728 ((Benzyl-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-etho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human Peroxisome proliferator activated receptor gamma | J Med Chem 48: 2248-50 (2005) Article DOI: 10.1021/jm0496436 BindingDB Entry DOI: 10.7270/Q2SX6CRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (PPAR alpha) (Homo sapiens (Human)) | BDBM50163728 ((Benzyl-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-etho...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human Peroxisome proliferator activated receptor alpha | J Med Chem 48: 2248-50 (2005) Article DOI: 10.1021/jm0496436 BindingDB Entry DOI: 10.7270/Q2SX6CRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (PPAR alpha) (Homo sapiens (Human)) | BDBM50163728 ((Benzyl-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-etho...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.02E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Agonist activity at PPARalpha receptor expressed in HEK293 cells by GAL4 transactivation assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50163728 ((Benzyl-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-etho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARgamma receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (PPAR alpha) (Homo sapiens (Human)) | BDBM50163728 ((Benzyl-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-etho...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50163728 ((Benzyl-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-etho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Agonist activity at PPARgamma receptor expressed in HEK293 cells by GAL4 transactivation assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (PPAR alpha) (Homo sapiens (Human)) | BDBM50163728 ((Benzyl-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-etho...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Effective concentration against human Peroxisome proliferator activated receptor alpha | J Med Chem 48: 2248-50 (2005) Article DOI: 10.1021/jm0496436 BindingDB Entry DOI: 10.7270/Q2SX6CRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||