

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50182680 CHEMBL373207::N-[4-[(3-chloro-4-fluorophenyl)amino]-6-quinazolinyl]-4-(diethylamino)-2-butynamide
SMILES: CCN(CC)CC#CC(=O)Nc1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1
InChI Key: InChIKey=FQGVUAQWOXXERW-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182680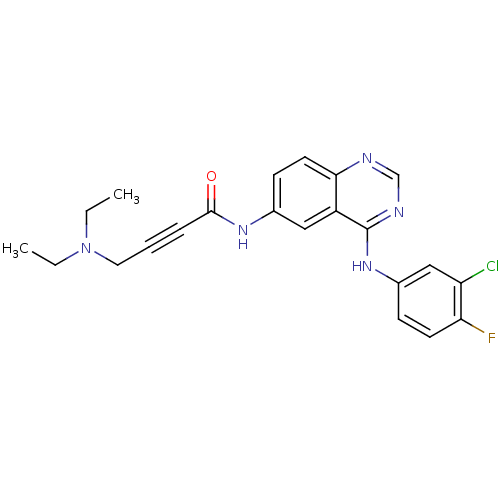 (CHEMBL373207 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of EGF stimulated human erbB1 autophosphorylation in NIH3T3 cells | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50182680 (CHEMBL373207 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB2 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50182680 (CHEMBL373207 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of HER stimulated human erbB autophosphorylation in MDA-MB-453 cells | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182680 (CHEMBL373207 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor protein-tyrosine kinase erbB-4 (Homo sapiens (Human)) | BDBM50182680 (CHEMBL373207 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB4 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||