

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50183263 CHEMBL3819434
SMILES: Cl.OC1(CC2CCC(C1)N2CCCCc1nc2ccccc2s1)c1ccc(Cl)cc1
InChI Key: InChIKey=SSUWLLVOHMVUDK-UHFFFAOYSA-N
Data: 19 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50183263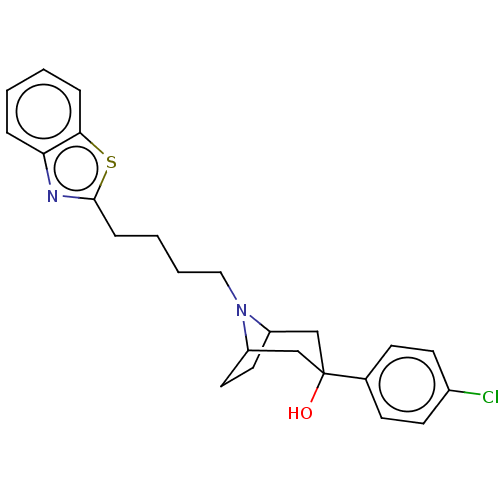 (CHEMBL3819434) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human recombinant dopamine D3 receptor incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50183263 (CHEMBL3819434) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human recombinant dopamine D3 receptor incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50183263 (CHEMBL3819434) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human recombinant dopamine D2 receptor incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50183263 (CHEMBL3819434) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human recombinant dopamine D2 receptor incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50183263 (CHEMBL3819434) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]Citalopram from human recombinant SERT incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50183263 (CHEMBL3819434) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]Citalopram from human recombinant SERT incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50183263 (CHEMBL3819434) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]Pyrilamine from human recombinant histamine H1 receptor incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50183263 (CHEMBL3819434) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]Pyrilamine from human recombinant histamine H1 receptor incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50183263 (CHEMBL3819434) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50183263 (CHEMBL3819434) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 274 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50183263 (CHEMBL3819434) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 339 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT2B receptor incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50183263 (CHEMBL3819434) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 342 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT2B receptor incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50183263 (CHEMBL3819434) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 432 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human recombinant dopamine D4 receptor incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50183263 (CHEMBL3819434) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human recombinant dopamine D4 receptor incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50183263 (CHEMBL3819434) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT7 receptor incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50183263 (CHEMBL3819434) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT7 receptor incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50183263 (CHEMBL3819434) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human recombinant 5HT2C receptor incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50183263 (CHEMBL3819434) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human recombinant 5HT2C receptor incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50183263 (CHEMBL3819434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University Curated by ChEMBL | Assay Description Displacement of [3H]Ketanserin from human recombinant 5HT2A receptor incubated for 1.5 hrs by microbeta scintillation counting method | Bioorg Med Chem 24: 3671-9 (2016) BindingDB Entry DOI: 10.7270/Q2W66NPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||