

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50188097 1-(4-Chlorobenzyl)-1H-imidazole::CHEMBL441367
SMILES: Clc1ccc(Cn2ccnc2)cc1
InChI Key: InChIKey=RPLWYOLCHGTNSX-UHFFFAOYSA-N
Data: 11 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50188097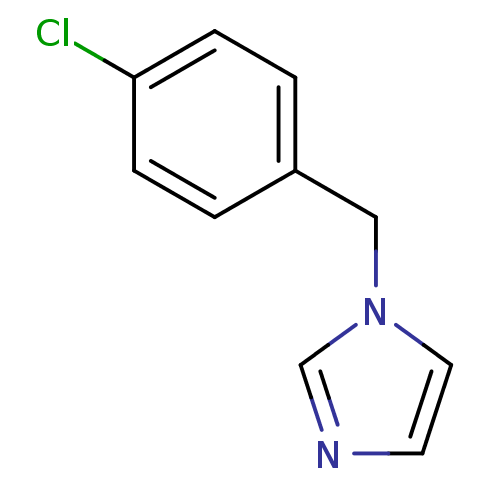 (1-(4-Chlorobenzyl)-1H-imidazole | CHEMBL441367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50188097 (1-(4-Chlorobenzyl)-1H-imidazole | CHEMBL441367) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 17A1 (Rattus norvegicus (Rat)) | BDBM50188097 (1-(4-Chlorobenzyl)-1H-imidazole | CHEMBL441367) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description Inhibition of rat microsomal 17,20-lyase component of P450-17alpha | Bioorg Med Chem Lett 16: 4011-5 (2006) Article DOI: 10.1016/j.bmcl.2006.05.070 BindingDB Entry DOI: 10.7270/Q27M07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 17A1 (Rattus norvegicus (Rat)) | BDBM50188097 (1-(4-Chlorobenzyl)-1H-imidazole | CHEMBL441367) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description Inhibition of rat microsomal 17alpha-hydroxylase component of P450-17alpha | Bioorg Med Chem Lett 16: 4011-5 (2006) Article DOI: 10.1016/j.bmcl.2006.05.070 BindingDB Entry DOI: 10.7270/Q27M07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 17A1 (Rattus norvegicus (Rat)) | BDBM50188097 (1-(4-Chlorobenzyl)-1H-imidazole | CHEMBL441367) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description Inhibition of rat testis 17alpha-hydroxylase component of P450-17alpha | Bioorg Med Chem Lett 16: 4752-6 (2006) Article DOI: 10.1016/j.bmcl.2006.06.092 BindingDB Entry DOI: 10.7270/Q2P55N43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50188097 (1-(4-Chlorobenzyl)-1H-imidazole | CHEMBL441367) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZ cells using 11-deoxycorticosterone substrate | ACS Med Chem Lett 2: 2-6 (2011) Article DOI: 10.1021/ml100071j BindingDB Entry DOI: 10.7270/Q28W3DKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 19A1 (Homo sapiens (Human)) | BDBM50188097 (1-(4-Chlorobenzyl)-1H-imidazole | CHEMBL441367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the West of Scotland Curated by ChEMBL | Assay Description Inhibition of aromatase | Bioorg Med Chem Lett 19: 4698-701 (2009) Article DOI: 10.1016/j.bmcl.2009.06.070 BindingDB Entry DOI: 10.7270/Q29K4B72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50188097 (1-(4-Chlorobenzyl)-1H-imidazole | CHEMBL441367) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eindhoven University of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in chinese hamster V79 cells | J Med Chem 53: 1712-25 (2010) Article DOI: 10.1021/jm901356d BindingDB Entry DOI: 10.7270/Q2XS5VGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2 (CYP11B2) (Homo sapiens (Human)) | BDBM50188097 (1-(4-Chlorobenzyl)-1H-imidazole | CHEMBL441367) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eindhoven University of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in chinese hamster V79 cells | J Med Chem 53: 1712-25 (2010) Article DOI: 10.1021/jm901356d BindingDB Entry DOI: 10.7270/Q2XS5VGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2 (CYP11B2) (Homo sapiens (Human)) | BDBM50188097 (1-(4-Chlorobenzyl)-1H-imidazole | CHEMBL441367) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZ cells using 11-deoxycorticosterone substrate | ACS Med Chem Lett 2: 2-6 (2011) Article DOI: 10.1021/ml100071j BindingDB Entry DOI: 10.7270/Q28W3DKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 17A1 (Rattus norvegicus (Rat)) | BDBM50188097 (1-(4-Chlorobenzyl)-1H-imidazole | CHEMBL441367) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description Inhibition of rat testis 17,20 lyase component of P450-17alpha | Bioorg Med Chem Lett 16: 4752-6 (2006) Article DOI: 10.1016/j.bmcl.2006.06.092 BindingDB Entry DOI: 10.7270/Q2P55N43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||