

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50190106 CHEMBL3827607::US10618914, Compound 32
SMILES: CC1(C)Sc2nnc(-c3[nH]nc4CCCc34)n2N=C1c1ccc(Cl)c(Cl)c1
InChI Key: InChIKey=OAVBVPBWKZMCDP-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50190106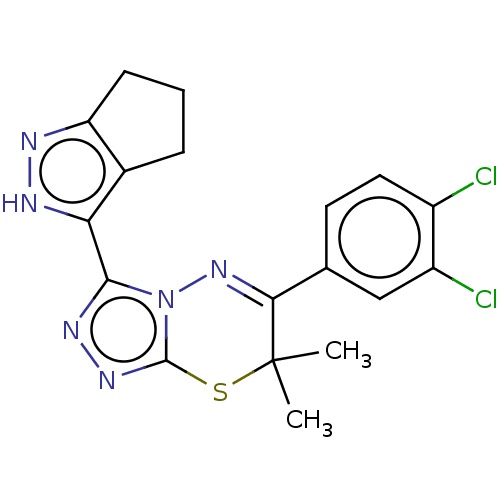 (CHEMBL3827607 | US10618914, Compound 32 | US111112...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Chemical Diversity Center Curated by ChEMBL | Assay Description Inhibition of IL6-induced STAT3 in human CAL33 cells | Bioorg Med Chem Lett 26: 3581-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.017 BindingDB Entry DOI: 10.7270/Q2ZK5JMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 1-alpha/beta (Homo sapiens (Human)) | BDBM50190106 (CHEMBL3827607 | US10618914, Compound 32 | US111112...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Chemical Diversity Center Curated by ChEMBL | Assay Description Inhibition of IFNgamma-induced STAT1 (unknown origin) | Bioorg Med Chem Lett 26: 3581-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.017 BindingDB Entry DOI: 10.7270/Q2ZK5JMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50190106 (CHEMBL3827607 | US10618914, Compound 32 | US111112...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 1-alpha/beta (Homo sapiens (Human)) | BDBM50190106 (CHEMBL3827607 | US10618914, Compound 32 | US111112...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Compound samples (in 3-5 mg quantities) were evaluated in a variety of assays. All compound submissions were fully characterized (1H, 13C, IR, HRMS),... | US Patent US10618914 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50190106 (CHEMBL3827607 | US10618914, Compound 32 | US111112...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh—Of the Commonwealth System of Higher Education US Patent | Assay Description Compound samples (in 3-5 mg quantities) were evaluated in a variety of assays. All compound submissions were fully characterized (1H, 13C, IR, HRMS),... | US Patent US10618914 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||