

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50192246 CHEMBL3935692
SMILES: Cc1ncoc1-c1nnc(SCCCN2CC[C@]3(C[C@@H]3c3ccc(cc3)C(F)(F)F)C2)n1C
InChI Key: InChIKey=MNAFRWGRFIBAQX-GCJKJVERSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50192246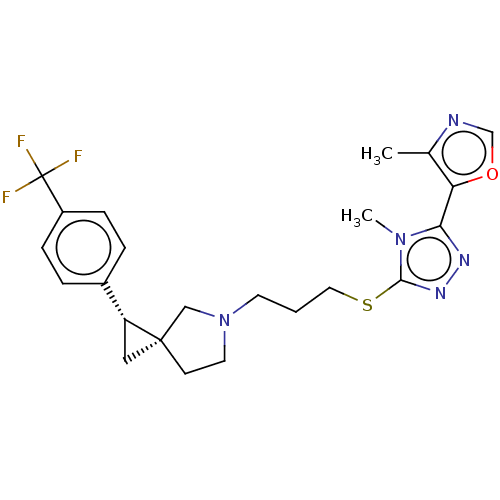 (CHEMBL3935692) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D3 receptor expressed in CHO-K1 cell membranes after 90 mins by liquid scintillation counting | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50192246 (CHEMBL3935692) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D3 receptor expressed in CHO cell membranes after 90 mins in presence of quinelorane by [35S]-GTPgammaS binding... | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50192246 (CHEMBL3935692) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D3 receptor expressed in CHO-K1 cell membranes after 90 mins by liquid scintillation counting | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50192246 (CHEMBL3935692) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D2L receptor expressed in CHO cells coexpressing Galpha16 assessed as inhibition of dopamine-induced Ca2+ stimu... | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50192246 (CHEMBL3935692) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2 receptor expressed in CHO-K1 cell membranes coexpressing Galpha16 after 120 mins by liquid scin... | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50192246 (CHEMBL3935692) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Activity at human muscarinic acetylcholine receptor M3 transfected in CHO-K1 cells assessed as intracellular calcium levels in presence of acetylchol... | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50192246 (CHEMBL3935692) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2 receptor expressed in CHO-K1 cell membranes coexpressing Galpha16 after 120 mins by liquid scin... | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50192246 (CHEMBL3935692) | UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 309 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Activity at human muscarinic acetylcholine receptor M1 transfected in CHO-K1 cells assessed as intracellular calcium levels in presence of acetylchol... | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50192246 (CHEMBL3935692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 expressed in microsomes using FCA as substrate after 10 mins by P450 cypex assay | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50192246 (CHEMBL3935692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in microsomes using 7BQ as substrate after 10 mins by P450 cypex assay | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50192246 (CHEMBL3935692) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 expressed in microsomes using ER as substrate after 10 mins by P450 cypex assay | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50192246 (CHEMBL3935692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 expressed in microsomes using BMC as substrate after 10 mins by P450 cypex assay | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50192246 (CHEMBL3935692) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in microsomes using MMC as substrate after 10 mins by P450 cypex assay | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50192246 (CHEMBL3935692) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 407 | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Inhibition of human ERG transfected in HEK293 cells assessed as reduction in tail current by patch clamp assay | J Med Chem 59: 8549-76 (2016) BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||