

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50197712 CHEMBL3676329::US10689705, Compound 44
SMILES: Cc1nc2cc(ccc2[nH]1)-n1ncc(C(=O)c2cc3ccc(Br)cc3[nH]2)c1N
InChI Key: InChIKey=AMMKZXLXQZTHBG-UHFFFAOYSA-N
Data: 10 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50197712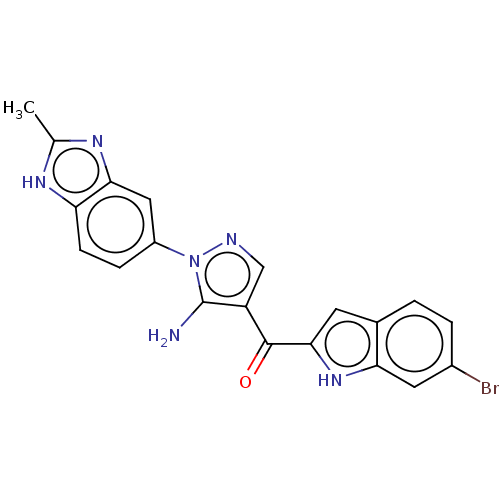 (CHEMBL3676329 | US10689705, Compound 44) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SRC by time-resolved fluorescence or time-resolved fluorescence assay | J Med Chem 59: 10586-10600 (2016) Article DOI: 10.1021/acs.jmedchem.6b01156 BindingDB Entry DOI: 10.7270/Q2ZS2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM50197712 (CHEMBL3676329 | US10689705, Compound 44) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human FGFR4 by time-resolved fluorescence or time-resolved fluorescence assay | J Med Chem 59: 10586-10600 (2016) Article DOI: 10.1021/acs.jmedchem.6b01156 BindingDB Entry DOI: 10.7270/Q2ZS2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM50197712 (CHEMBL3676329 | US10689705, Compound 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human FGFR3 by time-resolved fluorescence or time-resolved fluorescence assay | J Med Chem 59: 10586-10600 (2016) Article DOI: 10.1021/acs.jmedchem.6b01156 BindingDB Entry DOI: 10.7270/Q2ZS2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM50197712 (CHEMBL3676329 | US10689705, Compound 44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human FGFR2 by time-resolved fluorescence or time-resolved fluorescence assay | J Med Chem 59: 10586-10600 (2016) Article DOI: 10.1021/acs.jmedchem.6b01156 BindingDB Entry DOI: 10.7270/Q2ZS2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM50197712 (CHEMBL3676329 | US10689705, Compound 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha US Patent | Assay Description The FGFR3 inhibitory activities of compounds listed in Tables 1-1 to 1-5 were measured based on their activity to inhibit phosphorylation of the biot... | US Patent US10689705 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50197712 (CHEMBL3676329 | US10689705, Compound 44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human FGFR1 by time-resolved fluorescence or time-resolved fluorescence assay | J Med Chem 59: 10586-10600 (2016) Article DOI: 10.1021/acs.jmedchem.6b01156 BindingDB Entry DOI: 10.7270/Q2ZS2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GSC2 (Saccharomyces cerevisiae) | BDBM50197712 (CHEMBL3676329 | US10689705, Compound 44) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells assessed as reduction in channel currents at -80 mV holding potential by automated whole-cell patch cl... | J Med Chem 59: 10586-10600 (2016) Article DOI: 10.1021/acs.jmedchem.6b01156 BindingDB Entry DOI: 10.7270/Q2ZS2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50197712 (CHEMBL3676329 | US10689705, Compound 44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha US Patent | Assay Description The FGFR1 inhibitory activities of compounds were measured based on their activity to inhibit phosphorylation of the biotinylated peptide (EGPWLEEEEE... | US Patent US10689705 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM50197712 (CHEMBL3676329 | US10689705, Compound 44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha US Patent | Assay Description The FGFR2 inhibitory activities of compounds listed in Tables 1-1 to 1-5 were measured based on their activity to inhibit phosphorylation of the biot... | US Patent US10689705 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50197712 (CHEMBL3676329 | US10689705, Compound 44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human KDR by time-resolved fluorescence or time-resolved fluorescence assay | J Med Chem 59: 10586-10600 (2016) Article DOI: 10.1021/acs.jmedchem.6b01156 BindingDB Entry DOI: 10.7270/Q2ZS2ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||