

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50217556 CHEMBL125828
SMILES: O=C(N[C@@H]1N=C(c2ccccc2)c2cccc3CCN(c23)C1=O)c1cc2ccccc2cn1
InChI Key: InChIKey=MHRHYMTVTFSTHJ-VWLOTQADSA-N
Data: 7 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphodiesterase 4 (Homo sapiens (Human)) | BDBM50217556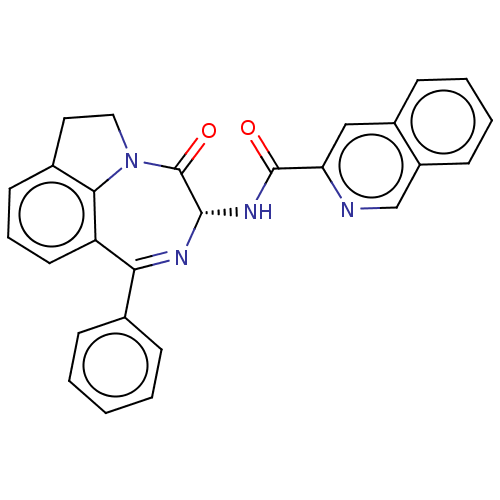 (CHEMBL125828) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Jouveinal-Parke Davis Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 4 extracted from human U937 cells by using radioligands [3H]-cAMP or [3H]cGMP. | Bioorg Med Chem Lett 10: 35-8 (2000) BindingDB Entry DOI: 10.7270/Q25Q4Z9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase 3 (Homo sapiens (Human)) | BDBM50217556 (CHEMBL125828) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Jouveinal-Parke Davis Curated by ChEMBL | Assay Description Inhibition of Phosphodiesterase 3 extracted from dog aorta smooth muscle cells by using radioligands [3H]cAMP or [3H]-cGMP. | Bioorg Med Chem Lett 10: 35-8 (2000) BindingDB Entry DOI: 10.7270/Q25Q4Z9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50217556 (CHEMBL125828) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Jouveinal-Parke Davis Curated by ChEMBL | Assay Description Binding affinity against high affinity rolipram binding site (HARBS) was determined by displacing radioactive [3H]rolipram from brain membrane suspen... | Bioorg Med Chem Lett 10: 35-8 (2000) BindingDB Entry DOI: 10.7270/Q25Q4Z9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase, PDE1/PDE5 (Homo sapiens (Human)) | BDBM50217556 (CHEMBL125828) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 1/5 isozyme (PDE1/5) mixture isolated from the guinea pig trachea. | J Med Chem 43: 4850-67 (2000) Article DOI: 10.1021/jm000315p BindingDB Entry DOI: 10.7270/Q25M68FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase 3 (Homo sapiens (Human)) | BDBM50217556 (CHEMBL125828) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 3 (PDE3) isolated from the dog aorta | J Med Chem 43: 4850-67 (2000) Article DOI: 10.1021/jm000315p BindingDB Entry DOI: 10.7270/Q25M68FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase 4 (Homo sapiens (Human)) | BDBM50217556 (CHEMBL125828) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase type 4 isozyme (PDE4) from the U937 human cell line. | J Med Chem 43: 4850-67 (2000) Article DOI: 10.1021/jm000315p BindingDB Entry DOI: 10.7270/Q25M68FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase, PDE1/PDE5 (Homo sapiens (Human)) | BDBM50217556 (CHEMBL125828) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Jouveinal-Parke Davis Curated by ChEMBL | Assay Description Inhibitory activity against phosphodiesterase 1/5 (PDE1/5) extracted from guinea pig trachea smooth muscle cells by using radioligands [3H]-cAMP or [... | Bioorg Med Chem Lett 10: 35-8 (2000) BindingDB Entry DOI: 10.7270/Q25Q4Z9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||