

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50220729 CHEMBL237320::N-((1s,4s)-4-hydroxy-4-(4-hydroxyphenyl)cyclohexyl)-3-phenylpropanamide
SMILES: Oc1ccc(cc1)[C@@]1(O)CC[C@@H](CC1)NC(=O)CCc1ccccc1
InChI Key: InChIKey=JIHJKNMBUYNINN-RVWIWJKTSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50220729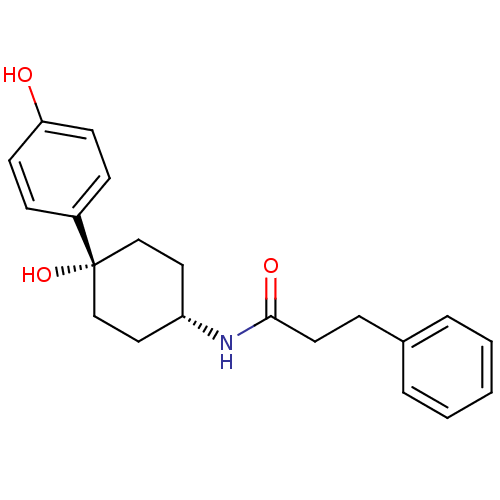 (CHEMBL237320 | N-((1s,4s)-4-hydroxy-4-(4-hydroxyph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human HERG expressed in HEK293 cells | Bioorg Med Chem Lett 17: 5533-6 (2007) Article DOI: 10.1016/j.bmcl.2007.08.039 BindingDB Entry DOI: 10.7270/Q23778FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220729 (CHEMBL237320 | N-((1s,4s)-4-hydroxy-4-(4-hydroxyph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane | Bioorg Med Chem Lett 17: 5533-6 (2007) Article DOI: 10.1016/j.bmcl.2007.08.039 BindingDB Entry DOI: 10.7270/Q23778FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||