

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50246380 2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-8-methoxy-3-methylquinazolin-4(3H)-one::CHEMBL453892
SMILES: COc1cccc2c1nc(-c1ccc(OC3CCN(CC3)C3CCC3)cc1)n(C)c2=O
InChI Key: InChIKey=MUZZBBKURZZINE-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246380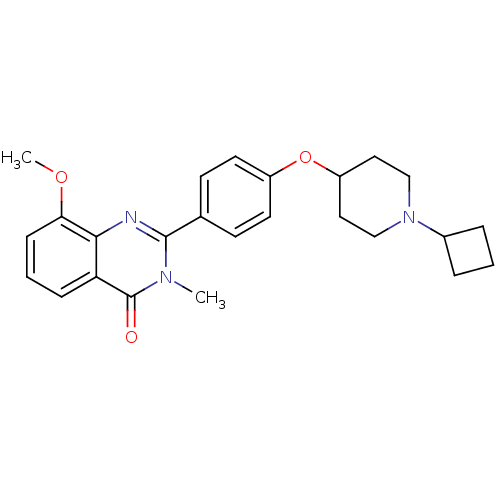 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-8-meth...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50246380 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-8-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S]MK499 from human ERG expressed in HEK293 cells | Bioorg Med Chem Lett 18: 6041-5 (2008) Article DOI: 10.1016/j.bmcl.2008.10.034 BindingDB Entry DOI: 10.7270/Q2QJ7H5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50246380 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-8-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human recombinant ERG by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246380 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-8-meth...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 6041-5 (2008) Article DOI: 10.1016/j.bmcl.2008.10.034 BindingDB Entry DOI: 10.7270/Q2QJ7H5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50246380 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-8-meth...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cells | Bioorg Med Chem Lett 18: 6041-5 (2008) Article DOI: 10.1016/j.bmcl.2008.10.034 BindingDB Entry DOI: 10.7270/Q2QJ7H5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||