

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50247539 CHEMBL472915::N-((3S,5S)-1-((E)-3-(3,4-dimethoxyphenyl)acryloyl)-5-(hydroxycarbamoyl)pyrrolidin-3-yl)nicotinamide::N-((3S,5S)-1-(3-(3,4-dimethoxyphenyl)acryloyl)-5-(hydroxycarbamoyl)pyrrolidin-3-yl)nicotinamide
SMILES: COc1ccc(\C=C\C(=O)N2C[C@H](C[C@H]2C(=O)NO)NC(=O)c2cccnc2)cc1OC
InChI Key: InChIKey=AKOSAZUQCGCWJD-IWDGFTLQSA-N
Data: 14 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50247539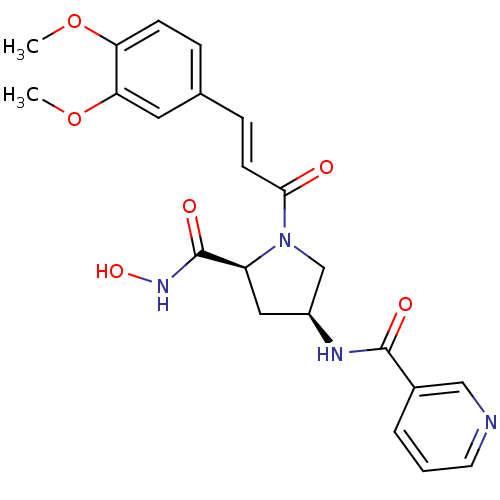 (CHEMBL472915 | N-((3S,5S)-1-((E)-3-(3,4-dimethoxyp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of pig kidney microsomal APN using L-Leu-p-nitroanilide as substrate preincubated for 5 mins before substrate addition measured after 30 m... | Bioorg Med Chem 22: 3055-64 (2014) Article DOI: 10.1016/j.bmc.2013.12.025 BindingDB Entry DOI: 10.7270/Q29888JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50247539 (CHEMBL472915 | N-((3S,5S)-1-((E)-3-(3,4-dimethoxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of MMP2 in human SKOV3 cell suspension using succinylated gelatin as substrate preincubated for 10 mins before substrate addition measured... | Bioorg Med Chem 22: 3055-64 (2014) Article DOI: 10.1016/j.bmc.2013.12.025 BindingDB Entry DOI: 10.7270/Q29888JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50247539 (CHEMBL472915 | N-((3S,5S)-1-((E)-3-(3,4-dimethoxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of MMP2 (unknown origin) using succinylated gelatin as substrate preincubated for 10 mins before substrate addition measured after 30 mins... | Bioorg Med Chem 22: 3055-64 (2014) Article DOI: 10.1016/j.bmc.2013.12.025 BindingDB Entry DOI: 10.7270/Q29888JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50247539 (CHEMBL472915 | N-((3S,5S)-1-((E)-3-(3,4-dimethoxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of MMP2 (unknown origin) by microtiter plate fluorimetric assay | Eur J Med Chem 43: 2130-9 (2008) Article DOI: 10.1016/j.ejmech.2007.12.020 BindingDB Entry DOI: 10.7270/Q22J6BNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50247539 (CHEMBL472915 | N-((3S,5S)-1-((E)-3-(3,4-dimethoxyp...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of aminopeptidase N (unknown origin) by microtiter plate fluorimetric assay | Eur J Med Chem 43: 2130-9 (2008) Article DOI: 10.1016/j.ejmech.2007.12.020 BindingDB Entry DOI: 10.7270/Q22J6BNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50247539 (CHEMBL472915 | N-((3S,5S)-1-((E)-3-(3,4-dimethoxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of MMP2 (unknown origin) | Bioorg Med Chem 16: 5398-404 (2008) Article DOI: 10.1016/j.bmc.2008.04.027 BindingDB Entry DOI: 10.7270/Q22B8XTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50247539 (CHEMBL472915 | N-((3S,5S)-1-((E)-3-(3,4-dimethoxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Weifang Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MMP9 (Phe107 to Pro449 residues) expressed in Escherichia coli preincubated for 45 mins followed by Mea-Pro-Leu-Gly-L... | Bioorg Med Chem 26: 4363-4374 (2018) Article DOI: 10.1016/j.bmc.2018.06.023 BindingDB Entry DOI: 10.7270/Q2251MV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50247539 (CHEMBL472915 | N-((3S,5S)-1-((E)-3-(3,4-dimethoxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of MMP2 (unknown origin) by microtiter plate fluorimetric assay | Bioorg Med Chem 16: 7932-8 (2008) Article DOI: 10.1016/j.bmc.2008.07.073 BindingDB Entry DOI: 10.7270/Q2MP533K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50247539 (CHEMBL472915 | N-((3S,5S)-1-((E)-3-(3,4-dimethoxyp...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of APN (unknown origin) by spectrophotometric assay | Bioorg Med Chem 16: 7932-8 (2008) Article DOI: 10.1016/j.bmc.2008.07.073 BindingDB Entry DOI: 10.7270/Q2MP533K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50247539 (CHEMBL472915 | N-((3S,5S)-1-((E)-3-(3,4-dimethoxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of gelatinase A (unknown origin) after 30 mins using succinylated gelatin as substrate | Bioorg Med Chem 24: 2125-36 (2016) BindingDB Entry DOI: 10.7270/Q28S4RSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50247539 (CHEMBL472915 | N-((3S,5S)-1-((E)-3-(3,4-dimethoxyp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of porcine microsomal aminopeptidase N preincubated for 30 mins using L-Leu-p-nitroanilide as substrate by UV-VIS spectrophotometer | Bioorg Med Chem 24: 2125-36 (2016) BindingDB Entry DOI: 10.7270/Q28S4RSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50247539 (CHEMBL472915 | N-((3S,5S)-1-((E)-3-(3,4-dimethoxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of MMP2 | Bioorg Med Chem 18: 1516-25 (2010) Article DOI: 10.1016/j.bmc.2010.01.008 BindingDB Entry DOI: 10.7270/Q2765FDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50247539 (CHEMBL472915 | N-((3S,5S)-1-((E)-3-(3,4-dimethoxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 266 | n/a | n/a | n/a | n/a | n/a | n/a |
Weifang Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human MMP2 catalytic domain (Tyr110 to Asp452 residues) preincubated for 45 mins followed by Mea-Pro-Leu-Gly-Leu-Dap(Dnp)-A... | Bioorg Med Chem 26: 4363-4374 (2018) Article DOI: 10.1016/j.bmc.2018.06.023 BindingDB Entry DOI: 10.7270/Q2251MV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50247539 (CHEMBL472915 | N-((3S,5S)-1-((E)-3-(3,4-dimethoxyp...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of aminopeptidase N (unknown origin) | Bioorg Med Chem 16: 5398-404 (2008) Article DOI: 10.1016/j.bmc.2008.04.027 BindingDB Entry DOI: 10.7270/Q22B8XTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||