

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: O=C1NCCc2nc(cc(Nc3cccc4ncccc34)c12)-c1ccccc1
InChI Key: InChIKey=KZRSALSNMARGNI-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50256133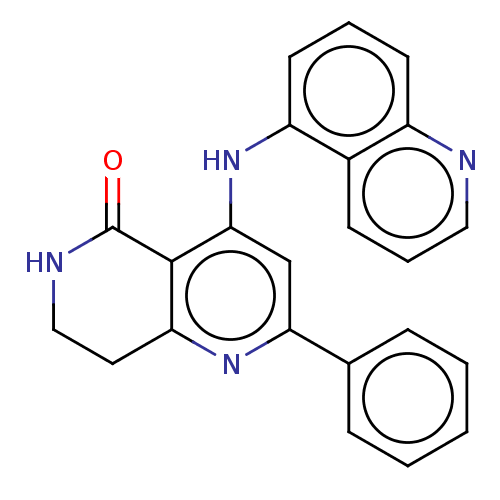 (CHEMBL4064980) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry& Screening ,?Pharmacokinetics& Metabolism , and§Experimental Dermatology , Almirall S.A., Centro de Investigación y Desarrollo , Crta. Laureà Miró 408-410 , Sant Feliu de Curated by ChEMBL | Assay Description Inhibition of recombinant human PDE4B1 using [3H]-cAMP as substrate preincubated for 15 mins followed substrate addition measured after 60 mins by sc... | J Med Chem 61: 2472-2489 (2018) Article DOI: 10.1021/acs.jmedchem.7b01751 BindingDB Entry DOI: 10.7270/Q2HQ42BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||