

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50266602 CHEMBL4061701
SMILES: Cl.Cl.CNCc1cc(CCc2ccc3c(C)cc(N)nc3c2)cc(c1)C#N
InChI Key: InChIKey=RWCOIXDPXHFMEG-UHFFFAOYSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric-oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50266602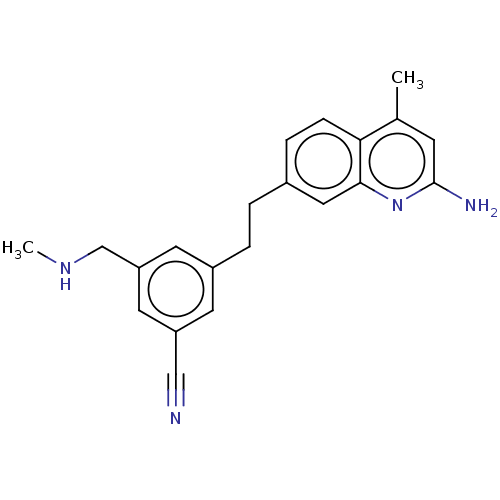 (CHEMBL4061701) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of recombinant rat nNOS expressed in Escherichia coli assessed as reduction in nitric oxide production using L-arginine as substrate measu... | J Med Chem 60: 3958-3978 (2017) Article DOI: 10.1021/acs.jmedchem.7b00259 BindingDB Entry DOI: 10.7270/Q2RV0R5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50266602 (CHEMBL4061701) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length nNOS expressed in Escherichia coli BL21(DE3) assessed as reduction in nitric oxide production using L-arg... | J Med Chem 60: 3958-3978 (2017) Article DOI: 10.1021/acs.jmedchem.7b00259 BindingDB Entry DOI: 10.7270/Q2RV0R5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50266602 (CHEMBL4061701) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of recombinant mouse macrophage iNOS expressed in Escherichia coli assessed as reduction in nitric oxide production using L-arginine as su... | J Med Chem 60: 3958-3978 (2017) Article DOI: 10.1021/acs.jmedchem.7b00259 BindingDB Entry DOI: 10.7270/Q2RV0R5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50266602 (CHEMBL4061701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length eNOS expressed in Escherichia coli BL21(DE3) assessed as reduction in nitric oxide production using L-arg... | J Med Chem 60: 3958-3978 (2017) Article DOI: 10.1021/acs.jmedchem.7b00259 BindingDB Entry DOI: 10.7270/Q2RV0R5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||