 Found 11 hits for monomerid = 50274648
Found 11 hits for monomerid = 50274648 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50274648
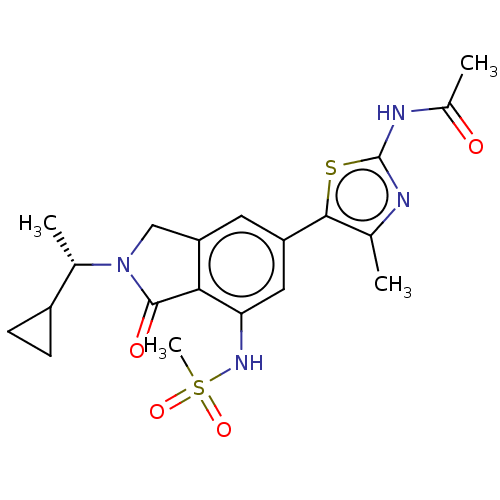
(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human BT474 cells assessed as reduction in Akt phosphorylation at Tyr308 residue after 2 hrs by fluorescence assay |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
p110α/p85α
(Homo sapiens (Human)) | BDBM50274648

(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3K p110alpha/p85alpha using DiC8-PIP2 as substrate preincubated for 10 mins followed by substrate addit... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
p110β/p85α
(Homo sapiens (Human)) | BDBM50274648

(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3K p110beta/p85alpha using DiC8-PIP2 as substrate preincubated for 10 mins followed by substrate additi... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphoinositide 3-Kinase (PI3K), delta
(Homo sapiens (Human)) | BDBM50274648

(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 10 mins followed by substrate addit... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50274648

(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274648

(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50274648

(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta in human JeKo1B cells assessed as reduction in AKT phosphorylation at ser473 residue preincubated for 60 mins followed by ant... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274648

(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase component GLS2
(Saccharomyces cerevisiae) | BDBM50274648

(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274648

(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50274648

(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in human MDA-MB-468 cells assessed as reduction in Akt phosphorylation at Tyr308 residue after 2 hrs |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data