

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50277548 CHEMBL4173394
SMILES: [Na;v0+].[#6]-[#6]-[#8]-c1ccc(-[#7]-c2ccc(cc2)-[#6](=[#6]-2\[#6]=[#6]/[#6](/[#6]=[#6]-2-[#6])=[#7+](/[#6]-[#6])-[#6]-c2cccc(c2)S([#8-])(=O)=O)\c2ccc(cc2-[#6])-[#7](-[#6]-[#6])-[#6]-c2cccc(c2)S([#8-])(=O)=O)cc1
InChI Key: InChIKey=YVNQAIFQFWTPLQ-UHFFFAOYSA-M
Data: 9 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50277548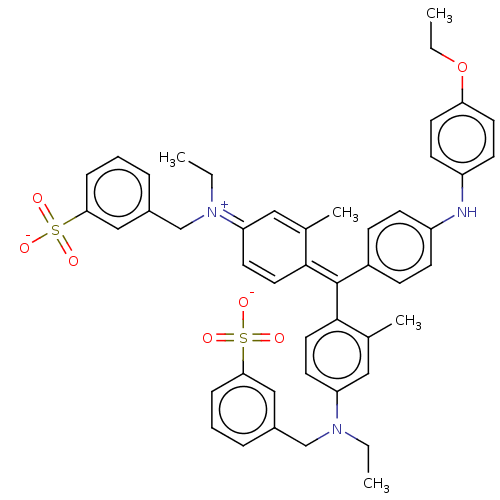 (CHEMBL4173394) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 536 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Oswaldo Cruz Curated by ChEMBL | Assay Description Antagonist activity at human P2X7R expressed in HEK293 cells assessed as inhibition of ATP-induced ethidium iodide uptake preincubated for 10 mins fo... | Eur J Med Chem 143: 1361-1372 (2018) Article DOI: 10.1016/j.ejmech.2017.10.033 BindingDB Entry DOI: 10.7270/Q2KS6V7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Mus musculus) | BDBM50277548 (CHEMBL4173394) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Oswaldo Cruz Curated by ChEMBL | Assay Description Antagonist activity at P2X7R in Swiss Webster mouse peritoneal macrophages assessed as inhibition of ATP-induced propidium iodide uptake preincubated... | Eur J Med Chem 143: 1361-1372 (2018) Article DOI: 10.1016/j.ejmech.2017.10.033 BindingDB Entry DOI: 10.7270/Q2KS6V7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-synuclein (Homo sapiens (Human)) | BDBM50277548 (CHEMBL4173394) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Immunology Curated by ChEMBL | Assay Description Inhibition of wild type alpha-synuclein (unknown origin) aggregation expressed in Escherichia coli BL21 after 30 days by thioflavin T staining-based ... | Eur J Med Chem 143: 1174-1184 (2018) Article DOI: 10.1016/j.ejmech.2017.10.002 BindingDB Entry DOI: 10.7270/Q2TB19JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Mus musculus) | BDBM50277548 (CHEMBL4173394) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 343 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description In vitro thromboxane A2 receptor antagonism through inhibition of U-46619 induced platelet aggregation in human whole blood | Eur J Med Chem 139: 698-717 (2017) Article DOI: 10.1016/j.ejmech.2017.08.034 BindingDB Entry DOI: 10.7270/Q2TX3HWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50277548 (CHEMBL4173394) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fluminense Federal University Curated by ChEMBL | Assay Description Antagonist activity at human P2X7R expressed in HEK293 cells assessed as inhibition of ATP-induced ethidium iodide uptake preincubated for 5 mins fol... | Bioorg Med Chem 27: 1449-1455 (2019) Article DOI: 10.1016/j.bmc.2018.11.036 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Mus musculus) | BDBM50277548 (CHEMBL4173394) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 407 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description In vitro thromboxane A2 receptor antagonism through inhibition of U-46619 induced platelet aggregation in human whole blood | Eur J Med Chem 139: 698-717 (2017) Article DOI: 10.1016/j.ejmech.2017.08.034 BindingDB Entry DOI: 10.7270/Q2TX3HWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Mus musculus) | BDBM50277548 (CHEMBL4173394) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in Swiss mouse peritoneal macrophages assessed as inhibition of ATP-induced current at holding potential of -60 ... | Eur J Med Chem 139: 698-717 (2017) Article DOI: 10.1016/j.ejmech.2017.08.034 BindingDB Entry DOI: 10.7270/Q2TX3HWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Mus musculus) | BDBM50277548 (CHEMBL4173394) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 432 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Compound was evaluated to inactivate the bacterial AdoMet-DC; value ranges from 3.8 to 39.6 uM | Eur J Med Chem 139: 698-717 (2017) Article DOI: 10.1016/j.ejmech.2017.08.034 BindingDB Entry DOI: 10.7270/Q2TX3HWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50277548 (CHEMBL4173394) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 552 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description In vitro thromboxane A2 receptor antagonism through inhibition of U-46619 induced contraction of rat isolated thoracic aortic strip | Eur J Med Chem 139: 698-717 (2017) Article DOI: 10.1016/j.ejmech.2017.08.034 BindingDB Entry DOI: 10.7270/Q2TX3HWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||