

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50282484 2-Ethyl-5,7-dimethyl-3-{5-[2-(1H-tetrazol-5-yl)-phenyl]-indan-1-yl}-3H-imidazo[4,5-b]pyridine::CHEMBL178380
SMILES: CCc1nc2c(C)cc(C)nc2n1C1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1
InChI Key: InChIKey=PACFDFGGIPMOKL-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin II receptor (Homo sapiens (Human)) | BDBM50282484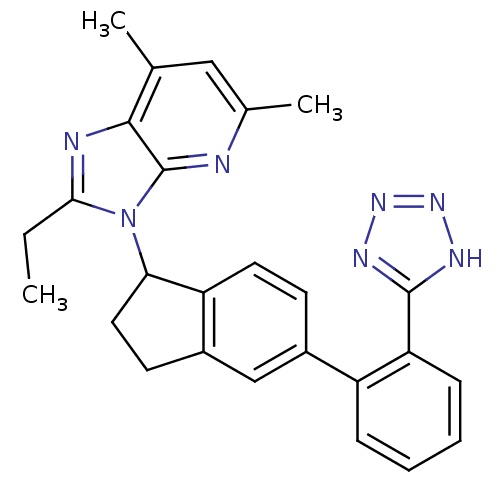 (2-Ethyl-5,7-dimethyl-3-{5-[2-(1H-tetrazol-5-yl)-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II receptor (AT-1) type-1 (RAT) | BDBM50282484 (2-Ethyl-5,7-dimethyl-3-{5-[2-(1H-tetrazol-5-yl)-ph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity against Angiotensin II receptor, type 1 using [125I]Sar-Ile8-AII in rat liver membranes | Citation and Details Article DOI: 10.1016/S0960-894X(01)81128-8 BindingDB Entry DOI: 10.7270/Q2TB19MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50282484 (2-Ethyl-5,7-dimethyl-3-{5-[2-(1H-tetrazol-5-yl)-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 574 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||