

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50285776 CHEMBL4172309
SMILES: [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)N1CCc2[nH]nnc2C1)C(=O)OCc1cc(cc(c1)C(=O)OC(F)(F)F)C#N
InChI Key: InChIKey=KLTYKZYRGMOAQZ-OSYLJGHBSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Rattus norvegicus) | BDBM50285776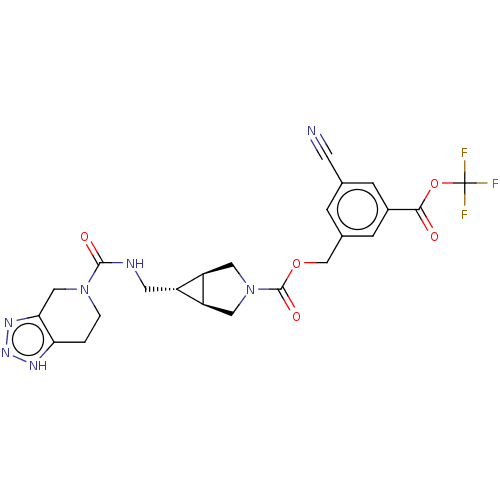 (CHEMBL4172309) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis | ACS Med Chem Lett 8: 1252-1257 (2017) Article DOI: 10.1021/acsmedchemlett.7b00312 BindingDB Entry DOI: 10.7270/Q2T43WNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50285776 (CHEMBL4172309) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells by patch clamp assay | ACS Med Chem Lett 8: 1252-1257 (2017) Article DOI: 10.1021/acsmedchemlett.7b00312 BindingDB Entry DOI: 10.7270/Q2T43WNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Rattus norvegicus) | BDBM50285776 (CHEMBL4172309) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of ATX in rat whole blood using LPA 17:0 as substrate after 1 hr by LC-MS/MS analysis | ACS Med Chem Lett 8: 1252-1257 (2017) Article DOI: 10.1021/acsmedchemlett.7b00312 BindingDB Entry DOI: 10.7270/Q2T43WNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||