

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50287266 CHEMBL290122::{3-[3-(3-Piperidin-1-ylmethyl-phenoxy)-propylcarbamoyl]-propyl}-carbamic acid 2-{3-[3-((R)-1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-ureido]-phenyl}-ethyl ester
SMILES: CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(CCOC(=O)NCCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1
InChI Key: InChIKey=UZFBINGRHVFVJI-WBCKFURZSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor (Mus musculus) | BDBM50287266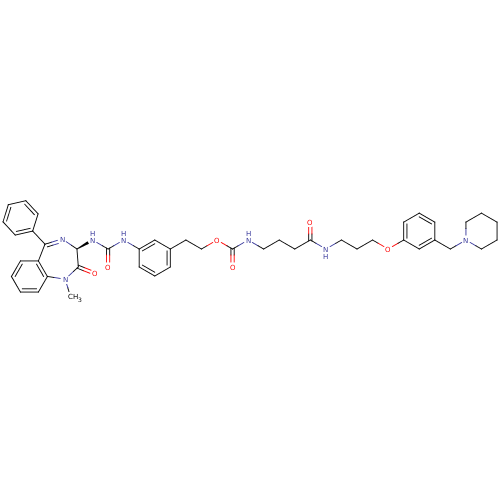 (CHEMBL290122 | {3-[3-(3-Piperidin-1-ylmethyl-pheno...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Cholecystokinin type A receptor | Bioorg Med Chem Lett 6: 1427-1430 (1996) Article DOI: 10.1016/S0960-894X(96)00249-1 BindingDB Entry DOI: 10.7270/Q2XD11NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (Homo sapiens (Human)) | BDBM50287266 (CHEMBL290122 | {3-[3-(3-Piperidin-1-ylmethyl-pheno...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against gastrin receptor. | Bioorg Med Chem Lett 6: 1427-1430 (1996) Article DOI: 10.1016/S0960-894X(96)00249-1 BindingDB Entry DOI: 10.7270/Q2XD11NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (MOUSE) | BDBM50287266 (CHEMBL290122 | {3-[3-(3-Piperidin-1-ylmethyl-pheno...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Cholecystokinin type B receptor | Bioorg Med Chem Lett 6: 1427-1430 (1996) Article DOI: 10.1016/S0960-894X(96)00249-1 BindingDB Entry DOI: 10.7270/Q2XD11NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||