

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50307380 CHEMBL589771::N-[(1S,2S,4R)-4-((S)-1-Benzylcarbamoyl-2-methyl-propylcarbamoyl)-1-(3,5-difluorophenoxymethyl)-2-hydroxy-4-methoxy-butyl]-5-(methanesulfonyl-methylamino)-N'-((R)-1-phenyl-ethyl)-isophthalamide
SMILES: CO[C@H](C[C@H](O)[C@H](COc1cc(F)cc(F)c1)NC(=O)c1cc(cc(c1)C(=O)N[C@H](C)c1ccccc1)N(C)CS(C)(=O)=O)C(=O)N[C@@H](C(C)C)C(=O)NCc1ccccc1
InChI Key: InChIKey=LNBSLHNHHTYHMF-GHRGXMHASA-N
Data: 2 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50307380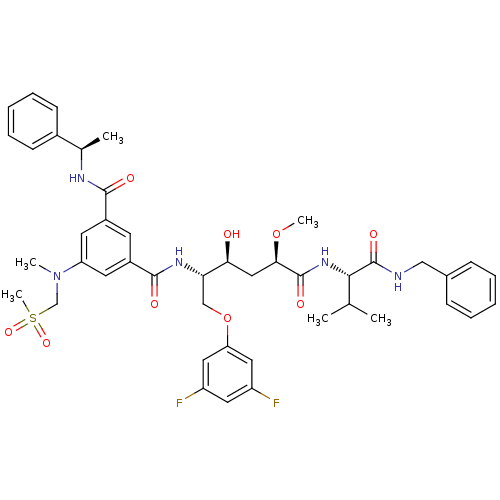 (CHEMBL589771 | N-[(1S,2S,4R)-4-((S)-1-Benzylcarbam...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stockholm University Curated by ChEMBL | Assay Description Inhibition of human BACE1 expressed in Escherichia coli cells (BL21(DE3) by TRF assay | J Med Chem 53: 1458-64 (2010) Article DOI: 10.1021/jm901168f BindingDB Entry DOI: 10.7270/Q2DR2VKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50307380 (CHEMBL589771 | N-[(1S,2S,4R)-4-((S)-1-Benzylcarbam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stockholm University Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin D | J Med Chem 53: 1458-64 (2010) Article DOI: 10.1021/jm901168f BindingDB Entry DOI: 10.7270/Q2DR2VKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||