

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50308188 2-(3-Hydroxy-4-methoxyanilino)-5,7-dihydro-6H-pyrimido[5,4-d]-[1]benzazepin-6-one::CHEMBL603463
SMILES: COc1ccc(Nc2ncc3CC(=O)Nc4ccccc4-c3n2)cc1O
InChI Key: InChIKey=SRLAWDYDLHMAMZ-UHFFFAOYSA-N
Data: 9 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase B (Homo sapiens (Human)) | BDBM50308188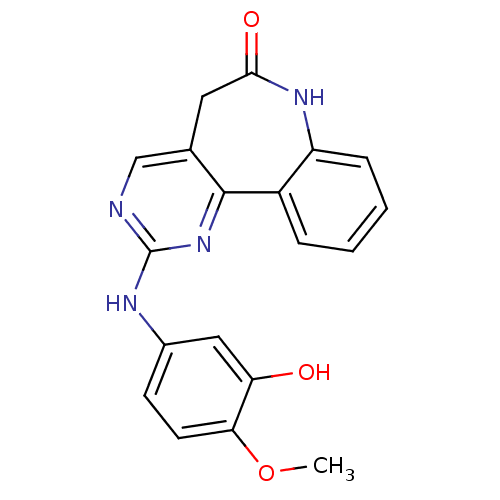 (2-(3-Hydroxy-4-methoxyanilino)-5,7-dihydro-6H-pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of recombinant Aurora B using tetra(LRRWSLG) as substrate assessed as inhibition of [33P]Phosphate incorporation into substrate after 80 m... | Eur J Med Chem 53: 254-63 (2012) Article DOI: 10.1016/j.ejmech.2012.04.007 BindingDB Entry DOI: 10.7270/Q2W66MT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50308188 (2-(3-Hydroxy-4-methoxyanilino)-5,7-dihydro-6H-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universitat Braunschweig Curated by ChEMBL | Assay Description Inhibition of PLK1 assessed as [33Pi] incorporation by microplate scintillation counting in presence of 1 uM ATP | J Med Chem 53: 2433-42 (2010) Article DOI: 10.1021/jm901388c BindingDB Entry DOI: 10.7270/Q24J0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50308188 (2-(3-Hydroxy-4-methoxyanilino)-5,7-dihydro-6H-pyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universitat Braunschweig Curated by ChEMBL | Assay Description Inhibition of INSR assessed as [33Pi] incorporation by microplate scintillation counting in presence of 1 uM ATP | J Med Chem 53: 2433-42 (2010) Article DOI: 10.1021/jm901388c BindingDB Entry DOI: 10.7270/Q24J0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50308188 (2-(3-Hydroxy-4-methoxyanilino)-5,7-dihydro-6H-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universitat Braunschweig Curated by ChEMBL | Assay Description Inhibition of VEGFR2 in HUE cells assessed as inhibition of VEGF-induced autophosphorylation treated for 90 mins before VEGF challenge by ELISA | J Med Chem 53: 2433-42 (2010) Article DOI: 10.1021/jm901388c BindingDB Entry DOI: 10.7270/Q24J0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50308188 (2-(3-Hydroxy-4-methoxyanilino)-5,7-dihydro-6H-pyri...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of recombinant AKT1 using GSK3 as substrate assessed as inhibition of [33P]Phosphate incorporation into substrate after 80 mins by scintil... | Eur J Med Chem 53: 254-63 (2012) Article DOI: 10.1016/j.ejmech.2012.04.007 BindingDB Entry DOI: 10.7270/Q2W66MT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50308188 (2-(3-Hydroxy-4-methoxyanilino)-5,7-dihydro-6H-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of recombinant FAK using poly(Glu,Tyr)4 as substrate assessed as inhibition of [33P]Phosphate incorporation into substrate after 80 mins b... | Eur J Med Chem 53: 254-63 (2012) Article DOI: 10.1016/j.ejmech.2012.04.007 BindingDB Entry DOI: 10.7270/Q2W66MT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50308188 (2-(3-Hydroxy-4-methoxyanilino)-5,7-dihydro-6H-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of recombinant VEGFR2 using poly(Glu,Tyr)4 as substrate assessed as inhibition of [33P]Phosphate incorporation into substrate after 80 min... | Eur J Med Chem 53: 254-63 (2012) Article DOI: 10.1016/j.ejmech.2012.04.007 BindingDB Entry DOI: 10.7270/Q2W66MT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50308188 (2-(3-Hydroxy-4-methoxyanilino)-5,7-dihydro-6H-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of recombinant PLK1 using RBERCHKtide as substrate assessed as inhibition of [33P]Phosphate incorporation into substrate after 80 mins by ... | Eur J Med Chem 53: 254-63 (2012) Article DOI: 10.1016/j.ejmech.2012.04.007 BindingDB Entry DOI: 10.7270/Q2W66MT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50308188 (2-(3-Hydroxy-4-methoxyanilino)-5,7-dihydro-6H-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universitat Braunschweig Curated by ChEMBL | Assay Description Inhibition of VEGFR2 assessed as [33Pi] incorporation by microplate scintillation counting in presence of 1 uM ATP | J Med Chem 53: 2433-42 (2010) Article DOI: 10.1021/jm901388c BindingDB Entry DOI: 10.7270/Q24J0G22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||