

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50320471 (4R,7S,10S,13S,16S,19R)-13-((1H-indol-3-yl)methyl)-19-acetamido-10-(4-aminobutyl)-16-benzyl-7-(naphthalen-1-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosane-4-carboxamide::CHEMBL1165796
SMILES: CC(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O
InChI Key: InChIKey=OGDNVDJQKDLFQU-SKGSPYGFSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UTS2R (RAT) | BDBM50320471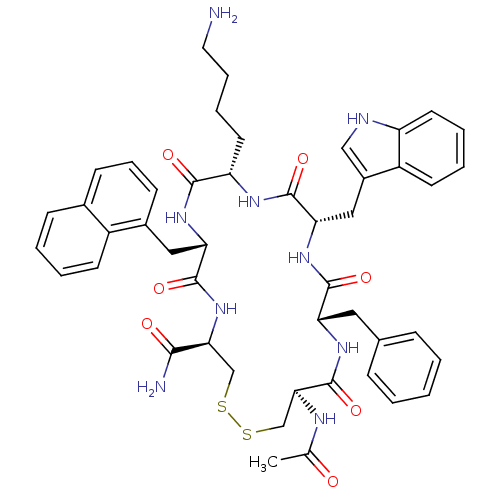 ((4R,7S,10S,13S,16S,19R)-13-((1H-indol-3-yl)methyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]U2 from rat urotensin 2 receptor expressed in CHO cells | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UTS2R (RAT) | BDBM50320471 ((4R,7S,10S,13S,16S,19R)-13-((1H-indol-3-yl)methyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization by FLIPR | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||