 Found 7 hits for monomerid = 50334764
Found 7 hits for monomerid = 50334764 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50334764
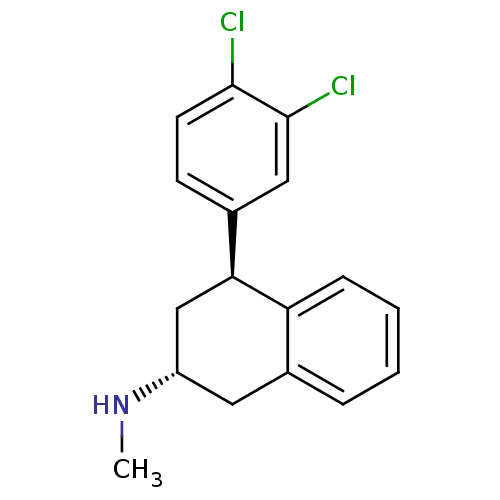
(CHEMBL1642894 | trans-(2R,4S)-4-(3,4-dichloropheny...)Show SMILES CN[C@@H]1C[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc2C1 |r| Show InChI InChI=1S/C17H17Cl2N/c1-20-13-8-11-4-2-3-5-14(11)15(10-13)12-6-7-16(18)17(19)9-12/h2-7,9,13,15,20H,8,10H2,1H3/t13-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]serotonin reuptake at human SERT expressed in HEK293 cells by scintillation counting |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50334764

(CHEMBL1642894 | trans-(2R,4S)-4-(3,4-dichloropheny...)Show SMILES CN[C@@H]1C[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc2C1 |r| Show InChI InChI=1S/C17H17Cl2N/c1-20-13-8-11-4-2-3-5-14(11)15(10-13)12-6-7-16(18)17(19)9-12/h2-7,9,13,15,20H,8,10H2,1H3/t13-,15-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]norepinephrine reuptake at human NET expressed in MDCK cells by scintillation counting |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50334764

(CHEMBL1642894 | trans-(2R,4S)-4-(3,4-dichloropheny...)Show SMILES CN[C@@H]1C[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc2C1 |r| Show InChI InChI=1S/C17H17Cl2N/c1-20-13-8-11-4-2-3-5-14(11)15(10-13)12-6-7-16(18)17(19)9-12/h2-7,9,13,15,20H,8,10H2,1H3/t13-,15-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]dopamine reuptake at human DAT expressed in CHOK1 cells by scintillation counting |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50334764

(CHEMBL1642894 | trans-(2R,4S)-4-(3,4-dichloropheny...)Show SMILES CN[C@@H]1C[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc2C1 |r| Show InChI InChI=1S/C17H17Cl2N/c1-20-13-8-11-4-2-3-5-14(11)15(10-13)12-6-7-16(18)17(19)9-12/h2-7,9,13,15,20H,8,10H2,1H3/t13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50334764

(CHEMBL1642894 | trans-(2R,4S)-4-(3,4-dichloropheny...)Show SMILES CN[C@@H]1C[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc2C1 |r| Show InChI InChI=1S/C17H17Cl2N/c1-20-13-8-11-4-2-3-5-14(11)15(10-13)12-6-7-16(18)17(19)9-12/h2-7,9,13,15,20H,8,10H2,1H3/t13-,15-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50334764

(CHEMBL1642894 | trans-(2R,4S)-4-(3,4-dichloropheny...)Show SMILES CN[C@@H]1C[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc2C1 |r| Show InChI InChI=1S/C17H17Cl2N/c1-20-13-8-11-4-2-3-5-14(11)15(10-13)12-6-7-16(18)17(19)9-12/h2-7,9,13,15,20H,8,10H2,1H3/t13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334764

(CHEMBL1642894 | trans-(2R,4S)-4-(3,4-dichloropheny...)Show SMILES CN[C@@H]1C[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc2C1 |r| Show InChI InChI=1S/C17H17Cl2N/c1-20-13-8-11-4-2-3-5-14(11)15(10-13)12-6-7-16(18)17(19)9-12/h2-7,9,13,15,20H,8,10H2,1H3/t13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem 19: 663-76 (2011)
Article DOI: 10.1016/j.bmc.2010.10.034
BindingDB Entry DOI: 10.7270/Q2639Q0M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data