

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50341269 (Z)-2-(4-Chlorophenyl)-5-(4-methoxybenzylidene)-5H-thiazol-4-one::CHEMBL1765899
SMILES: COc1ccc(\C=C2/SC(=NC2=O)c2ccc(Cl)cc2)cc1
InChI Key: InChIKey=BYPSEBXZQCBFIP-GDNBJRDFSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50341269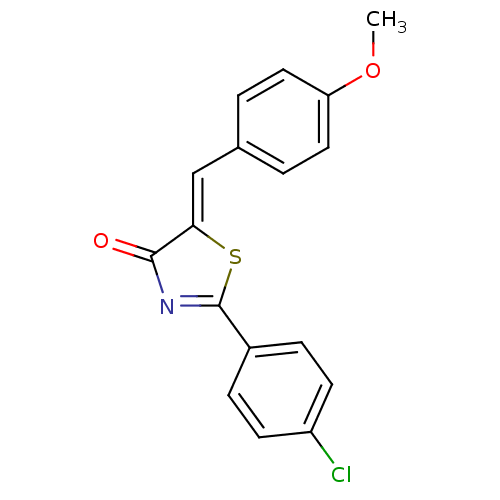 ((Z)-2-(4-Chlorophenyl)-5-(4-methoxybenzylidene)-5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human polymorphonuclear leukocytes assessed as inhibition of LTB4 production preincubated for 15 mins measured after ... | J Med Chem 54: 1943-7 (2011) Checked by Author Article DOI: 10.1021/jm101165z BindingDB Entry DOI: 10.7270/Q23T9J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50341269 ((Z)-2-(4-Chlorophenyl)-5-(4-methoxybenzylidene)-5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in S100 supernatant assessed as inhibition of (5(S)-hydroperoxy-6-trans-8,11,14-cis-eicosatetraenoic acid production pre... | J Med Chem 54: 1943-7 (2011) Checked by Author Article DOI: 10.1021/jm101165z BindingDB Entry DOI: 10.7270/Q23T9J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50341269 ((Z)-2-(4-Chlorophenyl)-5-(4-methoxybenzylidene)-5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in S100 supernatant assessed as inhibition of LTB4 production preincubated for 15 mins measured after 10 mins by HPLC me... | J Med Chem 54: 1943-7 (2011) Checked by Author Article DOI: 10.1021/jm101165z BindingDB Entry DOI: 10.7270/Q23T9J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50341269 ((Z)-2-(4-Chlorophenyl)-5-(4-methoxybenzylidene)-5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human polymorphonuclear leukocytes assessed as inhibition of (5(S)-hydroperoxy-6-trans-8,11,14-cis-eicosatetraenoic a... | J Med Chem 54: 1943-7 (2011) Checked by Author Article DOI: 10.1021/jm101165z BindingDB Entry DOI: 10.7270/Q23T9J70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||