

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50353395 CHEMBL1829782::US8592410, 95::US8592410, Comparator 10::US8598163, 68
SMILES: C[C@H](N1CC[C@@](CCCNS(C)(=O)=O)(OC1=O)c1ccc(F)cc1)c1ccc(cc1)-c1ccc(F)cc1F
InChI Key: InChIKey=SDPGWJVXVCTHJY-HMILPKGGSA-N
Data: 11 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353395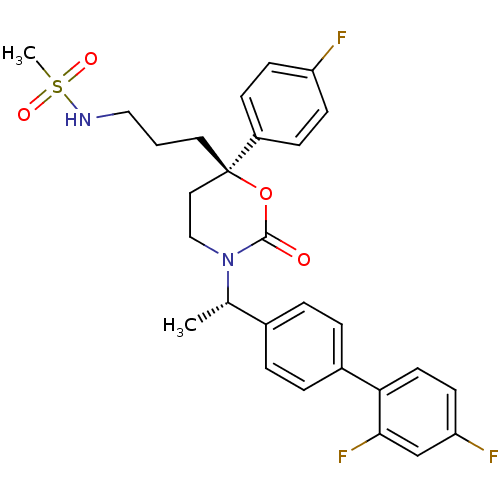 (CHEMBL1829782 | US8592410, 95 | US8592410, Compara...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.79 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8592410 (2013) BindingDB Entry DOI: 10.7270/Q2CF9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353395 (CHEMBL1829782 | US8592410, 95 | US8592410, Compara...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 53.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition assay using 11β-HSD1. | US Patent US8592410 (2013) BindingDB Entry DOI: 10.7270/Q2CF9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50353395 (CHEMBL1829782 | US8592410, 95 | US8592410, Compara...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of recombinant CYP2C9 by compounds of the invention was measured using a commercial kit from Invitrogen (cat #2859). | US Patent US8592410 (2013) BindingDB Entry DOI: 10.7270/Q2CF9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353395 (CHEMBL1829782 | US8592410, 95 | US8592410, Compara...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.79 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353395 (CHEMBL1829782 | US8592410, 95 | US8592410, Compara...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 53.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Inhibition assay using 11β-HSD1 in the presence of 50% human plasma. | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353395 (CHEMBL1829782 | US8592410, 95 | US8592410, Compara...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human 11 beta-HSD1 expressed in CHO cells assessed as conversion of [3H]-cortisone to [3H]-cortisol after 1 hr by scintillation proximi... | J Med Chem 54: 6050-62 (2011) Article DOI: 10.1021/jm2005354 BindingDB Entry DOI: 10.7270/Q2P26ZH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353395 (CHEMBL1829782 | US8592410, 95 | US8592410, Compara...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 53.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8598163 (2013) BindingDB Entry DOI: 10.7270/Q29K48WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353395 (CHEMBL1829782 | US8592410, 95 | US8592410, Compara...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of 11 beta-HSD1 in differentiated human adipocytes assessed as conversion of [3H]-cortisone to [3H]-cortisol after 10 mins by HPLC | J Med Chem 54: 6050-62 (2011) Article DOI: 10.1021/jm2005354 BindingDB Entry DOI: 10.7270/Q2P26ZH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353395 (CHEMBL1829782 | US8592410, 95 | US8592410, Compara...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human 11 beta-HSD1 expressed in CHO cells assessed as conversion of [3H]cortisone to [3H]cortisol after 1 hr by scintillation proximity... | J Med Chem 54: 6050-62 (2011) Article DOI: 10.1021/jm2005354 BindingDB Entry DOI: 10.7270/Q2P26ZH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50353395 (CHEMBL1829782 | US8592410, 95 | US8592410, Compara...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 assessed as rate of [14C]-formaldehyde/formic acid production by liquid scintillation counting | J Med Chem 54: 6050-62 (2011) Article DOI: 10.1021/jm2005354 BindingDB Entry DOI: 10.7270/Q2P26ZH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353395 (CHEMBL1829782 | US8592410, 95 | US8592410, Compara...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.79 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8598163 (2013) BindingDB Entry DOI: 10.7270/Q29K48WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||