

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1cc(c(OC)cc1-c1nc2sccn2c1\C=N\NC(N)=N)[N+]([O-])=O
InChI Key: InChIKey=DZYAMRZMUVLFTD-CNHKJKLMSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50355562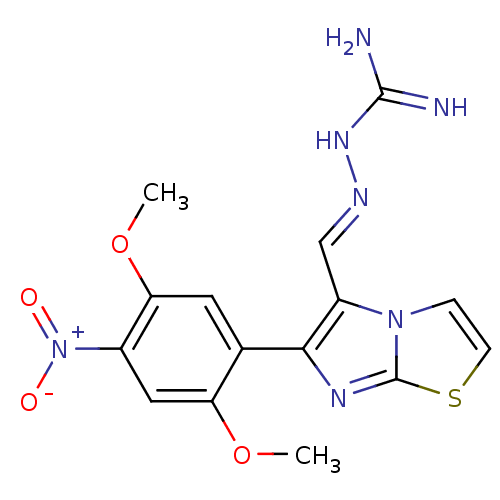 (CHEMBL1910630) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of CHK1 assessed as inhibition of substrate phosphorylation using fluorescent peptide substrate by microplate reader assay | Eur J Med Chem 46: 4311-23 (2011) Article DOI: 10.1016/j.ejmech.2011.07.001 BindingDB Entry DOI: 10.7270/Q2NG4R1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50355562 (CHEMBL1910630) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of CHK2 assessed as inhibition of substrate phosphorylation using fluorescent peptide substrate by microplate reader assay | Eur J Med Chem 46: 4311-23 (2011) Article DOI: 10.1016/j.ejmech.2011.07.001 BindingDB Entry DOI: 10.7270/Q2NG4R1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50355562 (CHEMBL1910630) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of RSK2 assessed as inhibition of substrate phosphorylation using fluorescent peptide substrate by microplate reader assay | Eur J Med Chem 46: 4311-23 (2011) Article DOI: 10.1016/j.ejmech.2011.07.001 BindingDB Entry DOI: 10.7270/Q2NG4R1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||