 Found 11 hits for monomerid = 50366237
Found 11 hits for monomerid = 50366237 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50366237
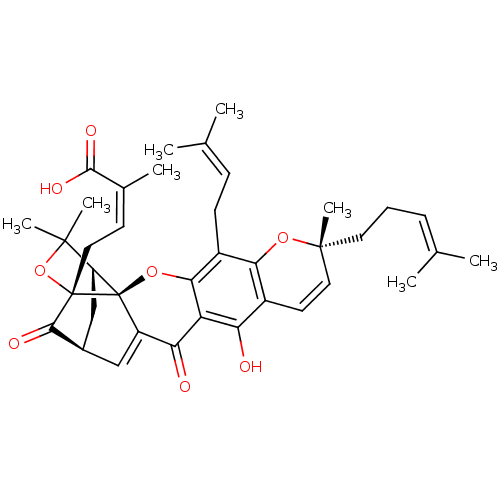
(GAMBOGIC ACID)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@@]1([#6])[#8]-c2c(-[#6]\[#6]=[#6](\[#6])-[#6])c3-[#8][C@@]45[#6@H]6-[#6]-[#6@@H](-[#6]=[#6]4-[#6](=O)-c3c(-[#8])c2-[#6]=[#6]1)-[#6](=O)[C@]5([#6]\[#6]=[#6](\[#6])-[#6](-[#8])=O)[#8]C6([#6])[#6] |r,c:22,33,TLB:22:21:18:42.43,THB:33:32:18:42.43,43:19:23.22:34.32| Show InChI InChI=1S/C38H44O8/c1-20(2)10-9-15-36(8)16-14-24-29(39)28-30(40)26-18-23-19-27-35(6,7)46-37(33(23)41,17-13-22(5)34(42)43)38(26,27)45-32(28)25(31(24)44-36)12-11-21(3)4/h10-11,13-14,16,18,23,27,39H,9,12,15,17,19H2,1-8H3,(H,42,43)/b22-13-/t23-,27+,36-,37+,38-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate by Dixon plot analysis |
Bioorg Med Chem 25: 2498-2506 (2017)
Article DOI: 10.1016/j.bmc.2017.03.010
BindingDB Entry DOI: 10.7270/Q2Q81GHS |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50366237

(GAMBOGIC ACID)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@@]1([#6])[#8]-c2c(-[#6]\[#6]=[#6](\[#6])-[#6])c3-[#8][C@@]45[#6@H]6-[#6]-[#6@@H](-[#6]=[#6]4-[#6](=O)-c3c(-[#8])c2-[#6]=[#6]1)-[#6](=O)[C@]5([#6]\[#6]=[#6](\[#6])-[#6](-[#8])=O)[#8]C6([#6])[#6] |r,c:22,33,TLB:22:21:18:42.43,THB:33:32:18:42.43,43:19:23.22:34.32| Show InChI InChI=1S/C38H44O8/c1-20(2)10-9-15-36(8)16-14-24-29(39)28-30(40)26-18-23-19-27-35(6,7)46-37(33(23)41,17-13-22(5)34(42)43)38(26,27)45-32(28)25(31(24)44-36)12-11-21(3)4/h10-11,13-14,16,18,23,27,39H,9,12,15,17,19H2,1-8H3,(H,42,43)/b22-13-/t23-,27+,36-,37+,38-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 499 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 60 mins followed by ... |
Bioorg Med Chem 25: 2498-2506 (2017)
Article DOI: 10.1016/j.bmc.2017.03.010
BindingDB Entry DOI: 10.7270/Q2Q81GHS |
More data for this
Ligand-Target Pair | |
Thioredoxin, mitochondrial
(Homo sapiens (Human)) | BDBM50366237

(GAMBOGIC ACID)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@@]1([#6])[#8]-c2c(-[#6]\[#6]=[#6](\[#6])-[#6])c3-[#8][C@@]45[#6@H]6-[#6]-[#6@@H](-[#6]=[#6]4-[#6](=O)-c3c(-[#8])c2-[#6]=[#6]1)-[#6](=O)[C@]5([#6]\[#6]=[#6](\[#6])-[#6](-[#8])=O)[#8]C6([#6])[#6] |r,c:22,33,TLB:22:21:18:42.43,THB:33:32:18:42.43,43:19:23.22:34.32| Show InChI InChI=1S/C38H44O8/c1-20(2)10-9-15-36(8)16-14-24-29(39)28-30(40)26-18-23-19-27-35(6,7)46-37(33(23)41,17-13-22(5)34(42)43)38(26,27)45-32(28)25(31(24)44-36)12-11-21(3)4/h10-11,13-14,16,18,23,27,39H,9,12,15,17,19H2,1-8H3,(H,42,43)/b22-13-/t23-,27+,36-,37+,38-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TRX-2 up to 60 mins by insulin reduction assay |
J Nat Prod 75: 1108-16 (2012)
Article DOI: 10.1021/np300118c
BindingDB Entry DOI: 10.7270/Q2NG4RS8 |
More data for this
Ligand-Target Pair | |
Thioredoxin
(Homo sapiens (Human)) | BDBM50366237

(GAMBOGIC ACID)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@@]1([#6])[#8]-c2c(-[#6]\[#6]=[#6](\[#6])-[#6])c3-[#8][C@@]45[#6@H]6-[#6]-[#6@@H](-[#6]=[#6]4-[#6](=O)-c3c(-[#8])c2-[#6]=[#6]1)-[#6](=O)[C@]5([#6]\[#6]=[#6](\[#6])-[#6](-[#8])=O)[#8]C6([#6])[#6] |r,c:22,33,TLB:22:21:18:42.43,THB:33:32:18:42.43,43:19:23.22:34.32| Show InChI InChI=1S/C38H44O8/c1-20(2)10-9-15-36(8)16-14-24-29(39)28-30(40)26-18-23-19-27-35(6,7)46-37(33(23)41,17-13-22(5)34(42)43)38(26,27)45-32(28)25(31(24)44-36)12-11-21(3)4/h10-11,13-14,16,18,23,27,39H,9,12,15,17,19H2,1-8H3,(H,42,43)/b22-13-/t23-,27+,36-,37+,38-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TRX-1 up to 60 mins by insulin reduction assay |
J Nat Prod 75: 1108-16 (2012)
Article DOI: 10.1021/np300118c
BindingDB Entry DOI: 10.7270/Q2NG4RS8 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50366237

(GAMBOGIC ACID)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@@]1([#6])[#8]-c2c(-[#6]\[#6]=[#6](\[#6])-[#6])c3-[#8][C@@]45[#6@H]6-[#6]-[#6@@H](-[#6]=[#6]4-[#6](=O)-c3c(-[#8])c2-[#6]=[#6]1)-[#6](=O)[C@]5([#6]\[#6]=[#6](\[#6])-[#6](-[#8])=O)[#8]C6([#6])[#6] |r,c:22,33,TLB:22:21:18:42.43,THB:33:32:18:42.43,43:19:23.22:34.32| Show InChI InChI=1S/C38H44O8/c1-20(2)10-9-15-36(8)16-14-24-29(39)28-30(40)26-18-23-19-27-35(6,7)46-37(33(23)41,17-13-22(5)34(42)43)38(26,27)45-32(28)25(31(24)44-36)12-11-21(3)4/h10-11,13-14,16,18,23,27,39H,9,12,15,17,19H2,1-8H3,(H,42,43)/b22-13-/t23-,27+,36-,37+,38-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of 6x-His tagged human recombinant full length Hsp90alpha ATPase preincubated for 0.5 hrs followed by ATP addition measured after 30 mins ... |
Bioorg Med Chem 24: 4626-4635 (2016)
Article DOI: 10.1016/j.bmc.2016.07.067 |
More data for this
Ligand-Target Pair | |
Heat Shock Protein 90 (Hsp90)
(Homo sapiens (Human)) | BDBM50366237

(GAMBOGIC ACID)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@@]1([#6])[#8]-c2c(-[#6]\[#6]=[#6](\[#6])-[#6])c3-[#8][C@@]45[#6@H]6-[#6]-[#6@@H](-[#6]=[#6]4-[#6](=O)-c3c(-[#8])c2-[#6]=[#6]1)-[#6](=O)[C@]5([#6]\[#6]=[#6](\[#6])-[#6](-[#8])=O)[#8]C6([#6])[#6] |r,c:22,33,TLB:22:21:18:42.43,THB:33:32:18:42.43,43:19:23.22:34.32| Show InChI InChI=1S/C38H44O8/c1-20(2)10-9-15-36(8)16-14-24-29(39)28-30(40)26-18-23-19-27-35(6,7)46-37(33(23)41,17-13-22(5)34(42)43)38(26,27)45-32(28)25(31(24)44-36)12-11-21(3)4/h10-11,13-14,16,18,23,27,39H,9,12,15,17,19H2,1-8H3,(H,42,43)/b22-13-/t23-,27+,36-,37+,38-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a |
Oklahoma State University
Curated by ChEMBL
| Assay Description
Binding affinity to human Hsp90 N-terminal domain (1 to 241) expressed in insect Sf9 cells by SPR analysis in presence of 20 uM geldanamycin |
J Nat Prod 74: 1085-92 (2011)
Article DOI: 10.1021/np200029q
BindingDB Entry DOI: 10.7270/Q2GB26XR |
More data for this
Ligand-Target Pair | |
Heat Shock Protein 90 (Hsp90)
(Homo sapiens (Human)) | BDBM50366237

(GAMBOGIC ACID)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@@]1([#6])[#8]-c2c(-[#6]\[#6]=[#6](\[#6])-[#6])c3-[#8][C@@]45[#6@H]6-[#6]-[#6@@H](-[#6]=[#6]4-[#6](=O)-c3c(-[#8])c2-[#6]=[#6]1)-[#6](=O)[C@]5([#6]\[#6]=[#6](\[#6])-[#6](-[#8])=O)[#8]C6([#6])[#6] |r,c:22,33,TLB:22:21:18:42.43,THB:33:32:18:42.43,43:19:23.22:34.32| Show InChI InChI=1S/C38H44O8/c1-20(2)10-9-15-36(8)16-14-24-29(39)28-30(40)26-18-23-19-27-35(6,7)46-37(33(23)41,17-13-22(5)34(42)43)38(26,27)45-32(28)25(31(24)44-36)12-11-21(3)4/h10-11,13-14,16,18,23,27,39H,9,12,15,17,19H2,1-8H3,(H,42,43)/b22-13-/t23-,27+,36-,37+,38-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a |
Oklahoma State University
Curated by ChEMBL
| Assay Description
Binding affinity to human full length Hsp90 expressed in insect Sf9 cells by SPR analysis |
J Nat Prod 74: 1085-92 (2011)
Article DOI: 10.1021/np200029q
BindingDB Entry DOI: 10.7270/Q2GB26XR |
More data for this
Ligand-Target Pair | |
Heat Shock Protein 90 (Hsp90)
(Homo sapiens (Human)) | BDBM50366237

(GAMBOGIC ACID)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@@]1([#6])[#8]-c2c(-[#6]\[#6]=[#6](\[#6])-[#6])c3-[#8][C@@]45[#6@H]6-[#6]-[#6@@H](-[#6]=[#6]4-[#6](=O)-c3c(-[#8])c2-[#6]=[#6]1)-[#6](=O)[C@]5([#6]\[#6]=[#6](\[#6])-[#6](-[#8])=O)[#8]C6([#6])[#6] |r,c:22,33,TLB:22:21:18:42.43,THB:33:32:18:42.43,43:19:23.22:34.32| Show InChI InChI=1S/C38H44O8/c1-20(2)10-9-15-36(8)16-14-24-29(39)28-30(40)26-18-23-19-27-35(6,7)46-37(33(23)41,17-13-22(5)34(42)43)38(26,27)45-32(28)25(31(24)44-36)12-11-21(3)4/h10-11,13-14,16,18,23,27,39H,9,12,15,17,19H2,1-8H3,(H,42,43)/b22-13-/t23-,27+,36-,37+,38-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a |
Oklahoma State University
Curated by ChEMBL
| Assay Description
Binding affinity to human Hsp90 N-terminal domain (1 to 241) expressed in insect Sf9 cells by SPR analysis |
J Nat Prod 74: 1085-92 (2011)
Article DOI: 10.1021/np200029q
BindingDB Entry DOI: 10.7270/Q2GB26XR |
More data for this
Ligand-Target Pair | |
Regulator of G-protein signaling 17
(Homo sapiens) | BDBM50366237

(GAMBOGIC ACID)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@@]1([#6])[#8]-c2c(-[#6]\[#6]=[#6](\[#6])-[#6])c3-[#8][C@@]45[#6@H]6-[#6]-[#6@@H](-[#6]=[#6]4-[#6](=O)-c3c(-[#8])c2-[#6]=[#6]1)-[#6](=O)[C@]5([#6]\[#6]=[#6](\[#6])-[#6](-[#8])=O)[#8]C6([#6])[#6] |r,c:22,33,TLB:22:21:18:42.43,THB:33:32:18:42.43,43:19:23.22:34.32| Show InChI InChI=1S/C38H44O8/c1-20(2)10-9-15-36(8)16-14-24-29(39)28-30(40)26-18-23-19-27-35(6,7)46-37(33(23)41,17-13-22(5)34(42)43)38(26,27)45-32(28)25(31(24)44-36)12-11-21(3)4/h10-11,13-14,16,18,23,27,39H,9,12,15,17,19H2,1-8H3,(H,42,43)/b22-13-/t23-,27+,36-,37+,38-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of RGS17 (unknown origin) GAP activity in presence of GTP by malachite green dye based assay |
J Nat Prod 80: 1992-2000 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00112
BindingDB Entry DOI: 10.7270/Q2RB777K |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50366237

(GAMBOGIC ACID)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@@]1([#6])[#8]-c2c(-[#6]\[#6]=[#6](\[#6])-[#6])c3-[#8][C@@]45[#6@H]6-[#6]-[#6@@H](-[#6]=[#6]4-[#6](=O)-c3c(-[#8])c2-[#6]=[#6]1)-[#6](=O)[C@]5([#6]\[#6]=[#6](\[#6])-[#6](-[#8])=O)[#8]C6([#6])[#6] |r,c:22,33,TLB:22:21:18:42.43,THB:33:32:18:42.43,43:19:23.22:34.32| Show InChI InChI=1S/C38H44O8/c1-20(2)10-9-15-36(8)16-14-24-29(39)28-30(40)26-18-23-19-27-35(6,7)46-37(33(23)41,17-13-22(5)34(42)43)38(26,27)45-32(28)25(31(24)44-36)12-11-21(3)4/h10-11,13-14,16,18,23,27,39H,9,12,15,17,19H2,1-8H3,(H,42,43)/b22-13-/t23-,27+,36-,37+,38-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate incubated for 10 mins measured for 30... |
Bioorg Med Chem 25: 2498-2506 (2017)
Article DOI: 10.1016/j.bmc.2017.03.010
BindingDB Entry DOI: 10.7270/Q2Q81GHS |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50366237

(GAMBOGIC ACID)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@@]1([#6])[#8]-c2c(-[#6]\[#6]=[#6](\[#6])-[#6])c3-[#8][C@@]45[#6@H]6-[#6]-[#6@@H](-[#6]=[#6]4-[#6](=O)-c3c(-[#8])c2-[#6]=[#6]1)-[#6](=O)[C@]5([#6]\[#6]=[#6](\[#6])-[#6](-[#8])=O)[#8]C6([#6])[#6] |r,c:22,33,TLB:22:21:18:42.43,THB:33:32:18:42.43,43:19:23.22:34.32| Show InChI InChI=1S/C38H44O8/c1-20(2)10-9-15-36(8)16-14-24-29(39)28-30(40)26-18-23-19-27-35(6,7)46-37(33(23)41,17-13-22(5)34(42)43)38(26,27)45-32(28)25(31(24)44-36)12-11-21(3)4/h10-11,13-14,16,18,23,27,39H,9,12,15,17,19H2,1-8H3,(H,42,43)/b22-13-/t23-,27+,36-,37+,38-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of IKK-beta using TMB substrate after 15 mins by spectrophotometer analysis |
Eur J Med Chem 51: 110-23 (2012)
Article DOI: 10.1016/j.ejmech.2012.02.029
BindingDB Entry DOI: 10.7270/Q27W6CPJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data