

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50367379 CHEMBL605239
SMILES: CCC(CC)Nc1ncnc2n(cnc12)C1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NC
InChI Key: InChIKey=KYOZXNLNQXFXES-USDBNLMRSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine A2 receptor (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50367379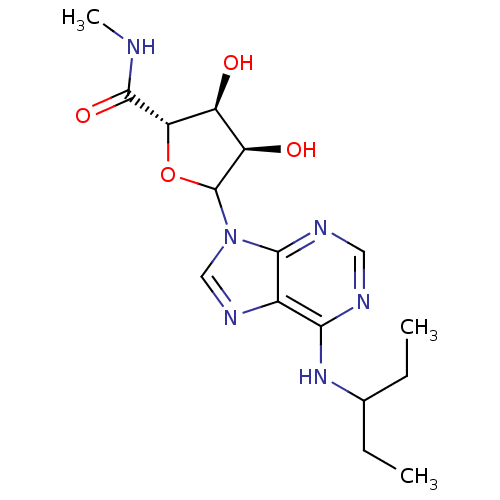 (CHEMBL605239) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Potency against PC12 cell A2 adenosine receptor by adenylate cyclase activation | J Med Chem 29: 1683-9 (1986) BindingDB Entry DOI: 10.7270/Q2Z89D0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50367379 (CHEMBL605239) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Potency against rat brain adenosine A1 receptor | J Med Chem 29: 1683-9 (1986) BindingDB Entry DOI: 10.7270/Q2Z89D0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||