

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCCCCCCCCCCCCCCC(=O)OCCCSC[C@H](NC(C)=O)C(=O)N[C@@H](CO)C(=O)OC
InChI Key: InChIKey=HUGFDRVKRYEYFN-DQEYMECFSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Toll-like receptor 2 (Homo sapiens (Human)) | BDBM50386536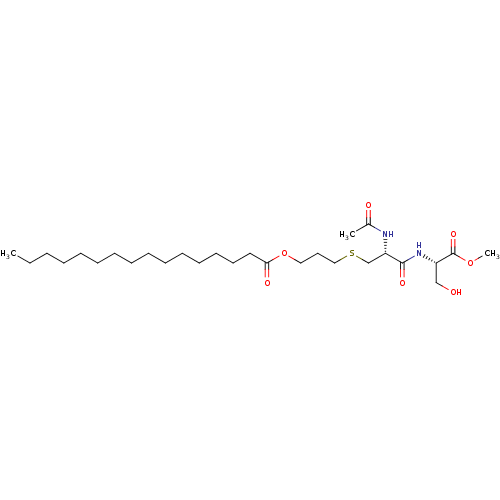 (CHEMBL2048220 | US9676818, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 5.52E+3 | n/a | n/a | n/a | n/a |
University of Kansas US Patent | Assay Description The induction of NF-κB in a TLR2-specific reporter gene assay was quantified using HEK-BLUE™ cells as previously described (Wu, W. et al., ... | US Patent US9676818 (2017) BindingDB Entry DOI: 10.7270/Q2TB1539 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 2 (Homo sapiens (Human)) | BDBM50386536 (CHEMBL2048220 | US9676818, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.52 | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human TLR2 expressed in HEK293 cells assessed as NFkappaB translocation after 12 hrs by secretory alkaline phosphatase reporter g... | J Med Chem 55: 3353-63 (2012) Article DOI: 10.1021/jm3000533 BindingDB Entry DOI: 10.7270/Q2JD4XT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||