

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50390673 CHEMBL2070198
SMILES: Brc1cccc(Nc2ncnc3ccc(NCc4ccc5OCCCOc5c4)cc23)c1
InChI Key: InChIKey=GGUUHEFKRMTXOW-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50390673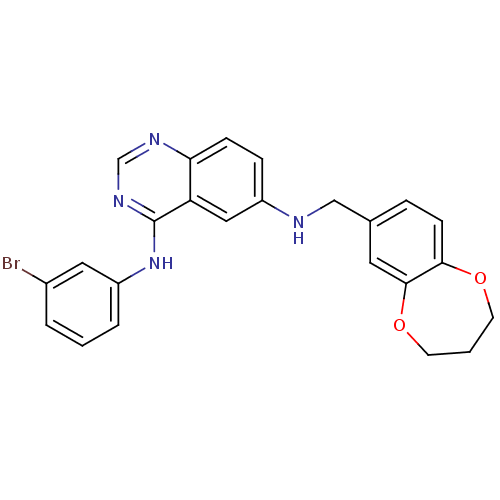 (CHEMBL2070198) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of EGFR in human A431 cell lysate using tyrosine/glutamic acid polymer as substrate by ELISA | Bioorg Med Chem Lett 22: 5870-5 (2012) Article DOI: 10.1016/j.bmcl.2012.07.079 BindingDB Entry DOI: 10.7270/Q26T0NRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50390673 (CHEMBL2070198) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of VEGFR2 using ATP incubated for 30 mins prior to ATP addition measured after 60 mins by fluorimetry | Bioorg Med Chem Lett 22: 5870-5 (2012) Article DOI: 10.1016/j.bmcl.2012.07.079 BindingDB Entry DOI: 10.7270/Q26T0NRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50390673 (CHEMBL2070198) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of recombinant HER2 expressed in baculovirus infected Sf9 cells using [32P]ATP incubated for 30 mins prior to ATP addition measured after ... | Bioorg Med Chem Lett 22: 5870-5 (2012) Article DOI: 10.1016/j.bmcl.2012.07.079 BindingDB Entry DOI: 10.7270/Q26T0NRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||