 Found 6 hits for monomerid = 50396097
Found 6 hits for monomerid = 50396097 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50396097
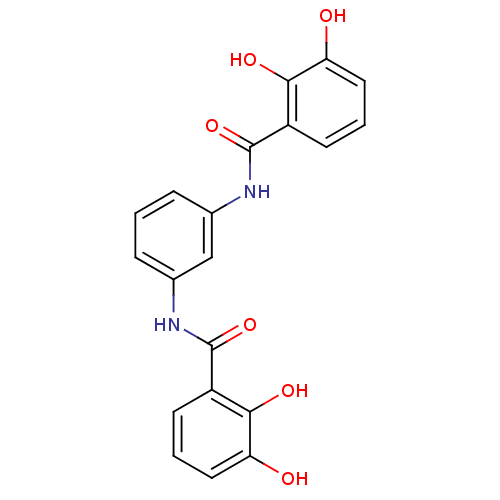
(CHEMBL2170856)Show SMILES Oc1cccc(C(=O)Nc2cccc(NC(=O)c3cccc(O)c3O)c2)c1O Show InChI InChI=1S/C20H16N2O6/c23-15-8-2-6-13(17(15)25)19(27)21-11-4-1-5-12(10-11)22-20(28)14-7-3-9-16(24)18(14)26/h1-10,23-26H,(H,21,27)(H,22,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of telomerase in human U937 cells by telomeric repeat amplification protocol |
J Med Chem 55: 3678-86 (2012)
Article DOI: 10.1021/jm201191d
BindingDB Entry DOI: 10.7270/Q2DF6SB3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50396097

(CHEMBL2170856)Show SMILES Oc1cccc(C(=O)Nc2cccc(NC(=O)c3cccc(O)c3O)c2)c1O Show InChI InChI=1S/C20H16N2O6/c23-15-8-2-6-13(17(15)25)19(27)21-11-4-1-5-12(10-11)22-20(28)14-7-3-9-16(24)18(14)26/h1-10,23-26H,(H,21,27)(H,22,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of telomerase in human HeLa cells using 5'-AAT CCG TCG AGC AGA GTT-3' as substrate incubated for 15 mins prior to extension reaction follo... |
J Med Chem 55: 3678-86 (2012)
Article DOI: 10.1021/jm201191d
BindingDB Entry DOI: 10.7270/Q2DF6SB3 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50396097

(CHEMBL2170856)Show SMILES Oc1cccc(C(=O)Nc2cccc(NC(=O)c3cccc(O)c3O)c2)c1O Show InChI InChI=1S/C20H16N2O6/c23-15-8-2-6-13(17(15)25)19(27)21-11-4-1-5-12(10-11)22-20(28)14-7-3-9-16(24)18(14)26/h1-10,23-26H,(H,21,27)(H,22,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity in human MDA-MB-435 cell lysate measured after 24 hrs by TRAP assay |
Eur J Med Chem 125: 117-129 (2017)
BindingDB Entry DOI: 10.7270/Q2NS0X5V |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50396097

(CHEMBL2170856)Show SMILES Oc1cccc(C(=O)Nc2cccc(NC(=O)c3cccc(O)c3O)c2)c1O Show InChI InChI=1S/C20H16N2O6/c23-15-8-2-6-13(17(15)25)19(27)21-11-4-1-5-12(10-11)22-20(28)14-7-3-9-16(24)18(14)26/h1-10,23-26H,(H,21,27)(H,22,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase isolated from human U937 cellular extracts by SYBR Green staining-based TRAP assay |
J Med Chem 57: 521-38 (2014)
Article DOI: 10.1021/jm400528t
BindingDB Entry DOI: 10.7270/Q2T43VKJ |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50396097

(CHEMBL2170856)Show SMILES Oc1cccc(C(=O)Nc2cccc(NC(=O)c3cccc(O)c3O)c2)c1O Show InChI InChI=1S/C20H16N2O6/c23-15-8-2-6-13(17(15)25)19(27)21-11-4-1-5-12(10-11)22-20(28)14-7-3-9-16(24)18(14)26/h1-10,23-26H,(H,21,27)(H,22,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity in human U2OS cell lysate measured after 24 hrs by TRAP assay |
Eur J Med Chem 125: 117-129 (2017)
BindingDB Entry DOI: 10.7270/Q2NS0X5V |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50396097

(CHEMBL2170856)Show SMILES Oc1cccc(C(=O)Nc2cccc(NC(=O)c3cccc(O)c3O)c2)c1O Show InChI InChI=1S/C20H16N2O6/c23-15-8-2-6-13(17(15)25)19(27)21-11-4-1-5-12(10-11)22-20(28)14-7-3-9-16(24)18(14)26/h1-10,23-26H,(H,21,27)(H,22,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of telomerase in human HeLa cells using 5'-AAT CCG TCG AGC AGA GTT-3' as substrate incubated for 15 mins prior to extension reaction by te... |
J Med Chem 55: 3678-86 (2012)
Article DOI: 10.1021/jm201191d
BindingDB Entry DOI: 10.7270/Q2DF6SB3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data