

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50397888 CHEMBL2179531
SMILES: Clc1ccoc1C(=O)N1CC2CNCC2C1
InChI Key: InChIKey=XXKYVKOMLITFKH-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor Alpha-4/Beta-2 (Homo sapiens (Human)) | BDBM50397888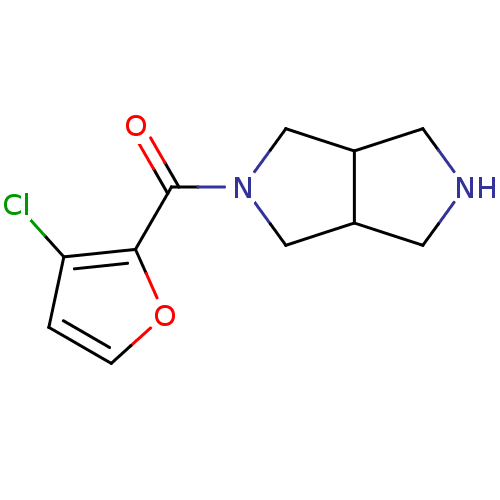 (CHEMBL2179531) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]nicotine from alpha4beta2 nAChR in human SH-EP1 cells after 2 hrs by liquid scintillation assay | J Med Chem 55: 9181-94 (2012) Article DOI: 10.1021/jm3006542 BindingDB Entry DOI: 10.7270/Q2W0972W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Homo sapiens (Human)) | BDBM50397888 (CHEMBL2179531) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 2.40 | -11.6 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc. US Patent | Assay Description The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... | US Patent US8921410 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor (Rattus norvegicus (Rat)) | BDBM50397888 (CHEMBL2179531) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 2.80 | -11.5 | n/a | n/a | n/a | n/a | n/a | n/a | 21 |
Targacept, Inc. US Patent | Assay Description The binding of [3H]MLA was measured using a modification of the methods of Davies et al., Neuropharmacol. 38: 679 (1999). [3H]MLA (Specific Activity=... | US Patent US8921410 (2014) BindingDB Entry DOI: 10.7270/Q2QZ28PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor protein alpha-7 subunit (Homo sapiens (Human)) | BDBM50397888 (CHEMBL2179531) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from human alpha7 nAChR expressed in CHO cells after 2 hrs by liquid scintillation counting | J Med Chem 55: 9181-94 (2012) Article DOI: 10.1021/jm3006542 BindingDB Entry DOI: 10.7270/Q2W0972W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor Alpha-4/Beta-2 (Homo sapiens (Human)) | BDBM50397888 (CHEMBL2179531) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a |
Targacept, Inc. Curated by ChEMBL | Assay Description Agonist activity at alpha4beta2 nAChR in human SH-EP1 cells assessed as calcium flux by calcium4-based FLIPR assay | J Med Chem 55: 9181-94 (2012) Article DOI: 10.1021/jm3006542 BindingDB Entry DOI: 10.7270/Q2W0972W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||