

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: C[Si](C)(C)c1cn(nn1)[C@H]1C[C@H](N(C1)C(=O)OCc1ccccc1)C(=O)N1CCCC1
InChI Key: InChIKey=AMUXFUKEWVWFMP-OALUTQOASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50399741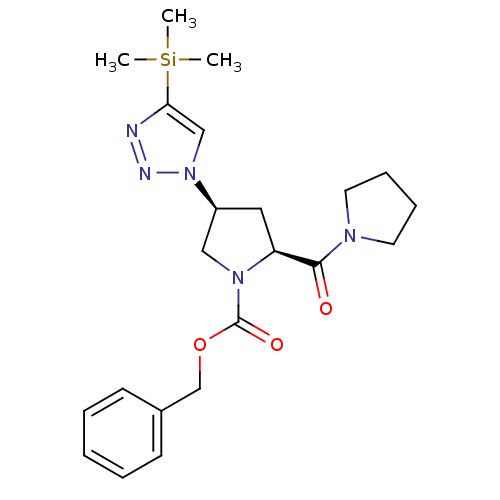 (CHEMBL2179405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human seminal plasma DPP4 assessed as pNA release from Gly-Pro-p-nitroanilide substrate pre-incubated with enzyme for 15 min prior to s... | J Med Chem 55: 9856-67 (2012) Article DOI: 10.1021/jm301060g BindingDB Entry DOI: 10.7270/Q2SN0B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Sus scrofa) | BDBM50399741 (CHEMBL2179405) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 956 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of pig PREP expressed in Escherichia coli using Z-Gly-Pro-p-nitroanilide substrate | J Med Chem 55: 9856-67 (2012) Article DOI: 10.1021/jm301060g BindingDB Entry DOI: 10.7270/Q2SN0B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Mus musculus (Mouse)) | BDBM50399741 (CHEMBL2179405) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of mouse recombinant FAP expressed in HEK293 cells assessed as pNA release from Ala-Pro-p-nitroanilide pre-incubated with enzyme for 15 mi... | J Med Chem 55: 9856-67 (2012) Article DOI: 10.1021/jm301060g BindingDB Entry DOI: 10.7270/Q2SN0B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50399741 (CHEMBL2179405) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human seminal plasma DPP2 assessed as pNA release from Lys-Ala-p-nitroanilide substrate pre-incubated with enzyme for 15 min prior to s... | J Med Chem 55: 9856-67 (2012) Article DOI: 10.1021/jm301060g BindingDB Entry DOI: 10.7270/Q2SN0B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||