

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Cc1nc2ccc(cc2[nH]1)[N+]([O-])=O
InChI Key: InChIKey=RKRXTVLCZDPERO-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2B1 (Rattus norvegicus) | BDBM50404900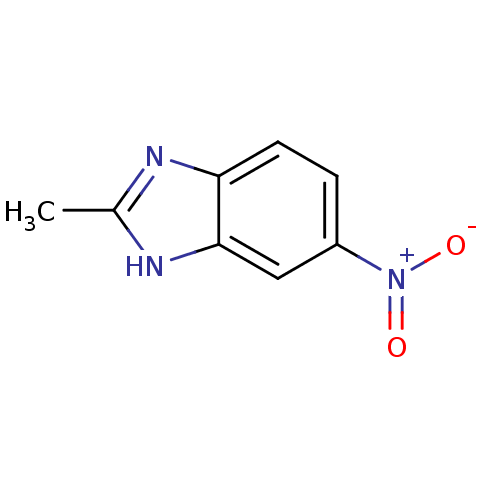 (CHEMBL353026) | PDB UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency to aminopyrine N-demethylase activity (P450) in hepatic microsomes from phenobarbitone-induced rats. | J Med Chem 25: 887-92 (1982) BindingDB Entry DOI: 10.7270/Q2VT1T8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||