

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50432603 CHEMBL2347187::US9296734, 59
SMILES: NC1=N[C@@](CF)(C[C@H](O1)C(F)(F)F)c1cc(NC(=O)c2ccc(cn2)C#N)ccc1F
InChI Key: InChIKey=QCMXWFUAVCHHIA-MAUKXSAKSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432603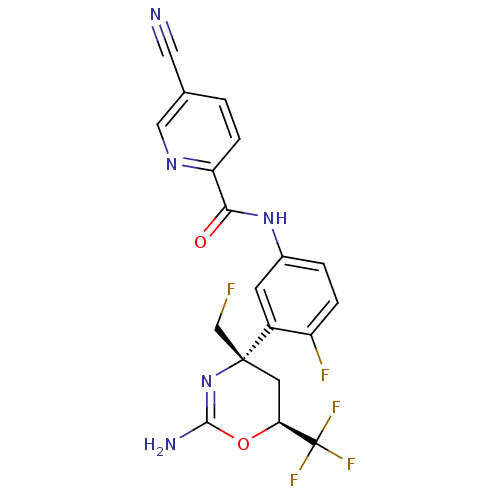 (CHEMBL2347187 | US9296734, 59) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | 37 |
Amgen Inc. US Patent | Assay Description The cell-based assay measures inhibition or reduction of Aβ40 in conditioned medium of test compound treated cells expressing amyloid precursor ... | US Patent US9296734 (2016) BindingDB Entry DOI: 10.7270/Q2DR2TB3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432603 (CHEMBL2347187 | US9296734, 59) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 17.9 | n/a | n/a | n/a | n/a | 4.2 | n/a |
Amgen Inc. US Patent | Assay Description The assay buffer used in this screen is 0.05 M acetate, pH 4.2, 10% DMSO final, 100 uM genapol (which is a nonionic detergent, below its Critical Mic... | US Patent US9296734 (2016) BindingDB Entry DOI: 10.7270/Q2DR2TB3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432603 (CHEMBL2347187 | US9296734, 59) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432603 (CHEMBL2347187 | US9296734, 59) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human BACE1 using MR121-labeled substrate incubated for 4 mins prior to substrate addition measured after 2 mins by spectrophotometric ... | J Med Chem 56: 3980-95 (2013) Article DOI: 10.1021/jm400225m BindingDB Entry DOI: 10.7270/Q2RX9DFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50432603 (CHEMBL2347187 | US9296734, 59) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | 3.5 | n/a |
Amgen Inc. US Patent | Assay Description Recombinant Cat D was expressed in CHO cells. The assay buffer for CathepsinD is 0.05 M citrate pH 3.5, 10% DMSO final, 5 mM CHAPS. The Cat D enzyme ... | US Patent US9296734 (2016) BindingDB Entry DOI: 10.7270/Q2DR2TB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||